Determination of Lithium-Ion Battery Capacity for Practical Applications
Abstract
:1. Introduction
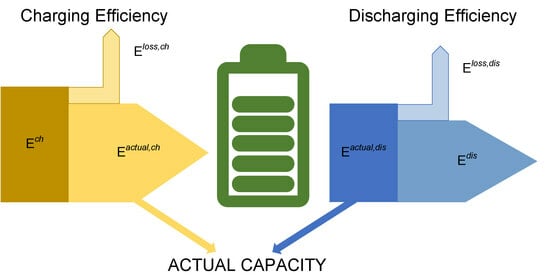
1.1. Battery Parameters
- Using heat loss measurements;
- Using open-circuit voltage vs. state-of-charge characteristics;
- using voltage/current measurements and the solution of the nonlinear optimization problem that consists of several measured round-trip efficiencies.
1.2. Literature Review
1.3. Contribution
- It proposes a method for determining battery capacity that considers charging/discharging (one-way) efficiencies, as well as different ambient temperatures;
- To verify the proposed method, an experimental comparison is performed to compare it with the baseline methods.
1.4. Organization of the Paper
2. Proposed Method for Determination of Average Battery Energy Capacity and State-of-Energy
3. Experimental Verification of the Proposed Method for Determination of Battery Energy Capacity and State-of-Energy
3.1. Experimental Setup
3.2. Compared Methods for Determination of Battery Energy Capacity and State-of-Energy
3.2.1. Method Nominal
3.2.2. Method Conventional
3.2.3. Method Proposed
3.3. Case Study
3.4. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Charging energy obtained by integration of | |
| Discharging energy obtained by integration of | |
| Average battery capacity estimated with method Nominal | |
| Average battery capacity estimated with method Conventional | |
| Average battery capacity estimated with method Proposed | |
| Charging energy obtained by integration of in method Proposed | |
| Discharging energy obtained by integration of in method Proposed | |
| Round-trip energy efficiency | |
| One-way charging energy efficiency | |
| One-way discharging energy efficiency | |
| Nominal round-trip energy efficiency defined by the manufacturer | |
| One-way charging energy efficiency in method Nominal | |
| One-way discharging energy efficiency in method Nominal | |
| Round-trip energy efficiency in method Conventional | |
| One-way charging energy efficiency in method Conventional | |
| One-way discharging energy efficiency in method Conventional | |
| One-way charging energy efficiency in method Proposed | |
| One-way discharging energy efficiency in method Proposed | |
| Charging power measured across battery terminals | |
| Discharging power measured across battery terminals | |
| Charging power corrected via efficiency–power characteristic in method Proposed | |
| Discharging power corrected via efficiency–power characteristics in method Proposed | |
| State-of-energy in method Nominal | |
| State-of-energy in method Conventional | |
| State-of-energy in method Proposed |
References
- Amir, S.; Gulzar, M.; Tarar, M.O.; Naqvi, I.H.; Zaffar, N.A.; Pecht, M.G. Dynamic Equivalent Circuit Model to Estimate State-of-Health of Lithium-Ion Batteries. IEEE Access 2022, 10, 18279–18288. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Handbook of Batteries; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Bobanac, V.; Bašić, H.; Pandžić, H. One-way voltaic and energy efficiency analysis for lithium-ion batteries. In Proceedings of the Medpower2022, The 13th Mediterranean Conference on Power Generation, Transmission, Distribution and Energy Conversion, Valletta, Malta, 7–9 November 2022; p. 53. [Google Scholar]
- Pandžić, H.; Bobanac, V.; Bašić, H. Method for Experimental Determination of Battery Parameters and Their Use. European Patent PCT/EP2022/070718, 2022. [Google Scholar]
- Farhad, S.; Nazari, A. Introducing the energy efficiency map of lithium-ion batteries. Int. J. Energy Res. 2018, 43, 931–944. [Google Scholar] [CrossRef]
- Lu, R.; Yang, A.; Xue, Y.; Xu, L.; Zhu, C. Analysis of the key factors affecting the energy efficiency of batteries in electric vehicle. World Electr. Veh. J. 2010, 4, 9–13. [Google Scholar] [CrossRef]
- Mamadou, K.; Lemaire, E.; Delaille, A.; Riu, D.; Hing, S.; Bultel, Y. Definition of a State-of-Energy Indicator (SoE) for Electrochemical Storage Devices: Application for Energetic Availability Forecasting. J. Electrochem. Soc. 2012, 159, A1298–A1307. [Google Scholar] [CrossRef]
- Pandžić, H.; Bobanac, V. An Accurate Charging Model of Battery Energy Storage. IEEE Trans. Power Syst. 2019, 34, 1416–1426. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, Z.; Gao, X. Coupling Analysis and Performance Study of Commercial 18650 Lithium-Ion Batteries under Conditions of Temperature and Vibration. Energies 2018, 11, 2856. [Google Scholar] [CrossRef]
- Xiong, R.; Cao, J.; Yu, Q.; He, H.; Sun, F. Critical Review on the Battery State of Charge Estimation Methods for Electric Vehicles. IEEE Access 2018, 6, 1832–1843. [Google Scholar] [CrossRef]
- Li, K.; Tseng, K.J. An equivalent circuit model for state of energy estimation of lithium-ion battery. In Proceedings of the 2016 IEEE Applied Power Electronics Conference and Exposition (APEC), Long Beach, CA, USA, 20–24 March 2016; pp. 3422–3430. [Google Scholar]
- Zheng, L.; Zhu, J.; Wang, G.; He, T.; Wei, Y. Novel methods for estimating lithium-ion battery state of energy and maximum available energy. Appl. Energy 2016, 178, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, W.; Ma, Z. Adaptive unscented Kalman filter based state of energy and power capability estimation approach for lithium-ion battery. J. Power Sources 2015, 289, 50–62. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Chen, Z. Model-based state-of-energy estimation of lithium-ion batteries in electric vehicles. Energy Procedia 2016, 88, 998–1004. [Google Scholar] [CrossRef]
- Dong, G.; Chen, Z.; Wei, J.; Zhang, C.; Wang, P. An online model-based method for state of energy estimation of lithium-ion batteries using dual filters. J. Power Sources 2016, 301, 277–286. [Google Scholar] [CrossRef]
- Li, K.; Tseng, K.J. Energy efficiency of lithium-ion battery used as energy storage devices in micro-grid. In Proceedings of the IECON 2015-41st Annual Conference of the IEEE Industrial Electronics Society, Yokohama, Japan, 9–12 November 2015; pp. 005235–005240. [Google Scholar]
- Bhat, C.; Channegowda, J.; Naraharisetti, K. Electrolyte based Equivalent Circuit Model of Lithium ion Batteries for Intermittent Load Applications. In Proceedings of the 2022 IEEE International Conference on Power Electronics, Smart Grid, and Renewable Energy (PESGRE), Trivandrum, India, 2–5 January 2022; pp. 1–3. [Google Scholar]
- Bhagyasree, P.; Shah, V.A. A Simplified Method to Evaluate Equivalent Circuit Model and State of Charge of Li-ion Battery. In Proceedings of the 2019 IEEE 1st International Conference on Energy, Systems and Information Processing (ICESIP), Chennai, India, 4–6 July 2019; pp. 1–6. [Google Scholar]
- Fonseca, J.M.L.; Sambandam Kulothungan, G.; Raj, K.; Rajashekara, K. A Novel State of Charge Dependent Equivalent Circuit Model Parameter Offline Estimation for Lithium-ion Batteries in Grid Energy Storage Applications. In Proceedings of the 2020 IEEE Industry Applications Society Annual Meeting, Detroit, MI, USA, 10–16 October 2020; pp. 1–8. [Google Scholar]
- Ko, Y.; Cho, K.; Kim, M.; Choi, W. A Novel Capacity Estimation Method for the Lithium Batteries Using the Enhanced Coulomb Counting Method with Kalman Filtering. IEEE Access 2022, 10, 38793–38801. [Google Scholar] [CrossRef]
- An, F.; Jiang, J.; Zhang, W.; Zhang, C.; Fan, X. State of Energy Estimation for Lithium-Ion Battery Pack via Prediction in Electric Vehicle Applications. IEEE Trans. Veh. Technol. 2022, 71, 184–195. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kok Soon, T.; Bin Idris, M.Y.I.; Mekhilef, S.; Adnan, S.B.R.S. Combined State of Charge and state-of-energy Estimation of Lithium-Ion Battery Using Dual Forgetting Factor-Based Adaptive Extended Kalman Filter for Electric Vehicle Applications. IEEE Trans. Veh. Technol. 2022, 70, 1200–1215. [Google Scholar] [CrossRef]
- Plett, G.L. Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs: Part 3. State and parameter estimation. J. Power Sources 2004, 134, 277–292. [Google Scholar] [CrossRef]
- Mastali, M.; Vazquez-Arenas, J.; Fraser, R.; Fowler, M.; Afshar, S.; Stevens, M. Battery state of the charge estimation using Kalman filtering. J. Power Sources 2013, 239, 294–307. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, Y.; Mi, C.C. State of charge estimation of lithium-ion batteries in electric drive vehicles using extended Kalman filtering. IEEE Trans. Veh. Technol. 2013, 62, 1020–1030. [Google Scholar] [CrossRef]
- Liu, G.; Ouyang, M.; Lu, L.; Li, J.; Hua, J. A highly accurate predictive-adaptive method for lithium-ion battery remaining discharge energy prediction in electric vehicle applications. Appl. Energy 2015, 149, 297–314. [Google Scholar] [CrossRef]
- Ren, D.; Lu, L.; Shen, P.; Feng, X.; Han, X.; Ouyang, M. Battery remaining discharge energy estimation based on prediction of future operating conditions. J. Energy Storage 2019, 25, 100836. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Zhang, C.; Chen, Z. A method for state of energy estimation of lithium-ion batteries at dynamic currents and temperatures. J. Power Sources 2014, 270, 151–157. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, X.; Zhang, C.; Chen, Z. A method for state of energy estimation of lithium-ion batteries based on neural network model. Energy 2015, 90, 879–888. [Google Scholar] [CrossRef]
- Singh, P.; Fennie, C.; Reisner, D. Fuzzy logic modelling of state-of-charge and available capacity of nickel/metal hydride batteries. J. Power Sources 2004, 136, 322–333. [Google Scholar] [CrossRef]
- Choi, Y.; Ryu, S.; Park, K.; Kim, H. Machine Learning-Based Lithium-Ion Battery Capacity Estimation Exploiting Multi-Channel Charging Profiles. IEEE Access 2019, 7, 75143–75152. [Google Scholar] [CrossRef]
- Lucu, M.; Martinez-Laserna, E.; Gandiaga, I.; Camblong, H. A critical review on self-adaptive Li-ion battery ageing models. J. Power Sources 2018, 401, 85–101. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Q.; Bi, Y.; Cai, M.; Dunn, B.; Glossmann, T.; Liu, J.; Osaka, T.; Sugiura, R.; Wu, B.; et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 2020, 5, 561–568. [Google Scholar] [CrossRef]
- Itech, IT-M3400 Bidirectional DC Power Supply. Available online: https://www.itechate.com/uploadfiles/ (accessed on 23 December 2021).







| Battery Cells | NMC | |
|---|---|---|
| Parameter | ||
| Type | 18,650 | |
| Nominal capacity | 3.0 Ah | |
| Nominal energy capacity | 10.8 Wh | |
| Nominal voltage | 3.6 V | |
| Charging voltage | 4.2 V | |
| Discharge cut-off voltage | 2.5 V | |
| Cut-off current | 0.05 A | |
| Max. charge current | 1.33 C | |
| Max. discharge current | 6.67 C | |
| Temperature | 0 °C | 25 °C | |
|---|---|---|---|
| Method | |||
| Nominal | 8.64 Wh | 10.80 Wh | |
| Conventional | 9.86 Wh | 10.53 Wh | |
| Proposed | 10.05 Wh | 10.68 Wh | |
| P-Rate | 0.0P | 0.1P | 0.2P | 0.3P | 0.4P | 0.5P | 0.6P | 0.7P | 0.8P | 0.9P | 1.0P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conditions | ||||||||||||
| Charging at 0 °C | 1 | 0.985 | 0.977 | 0.970 | 0.964 | 0.959 | 0.954 | 0.949 | 0.944 | 0.940 | 0.935 | |
| Discharging at 0 °C | 1 | 0.983 | 0.974 | 0.965 | 0.958 | 0.951 | 0.944 | 0.938 | 0.932 | 0.927 | 0.921 | |
| Charging at 25 °C | 1 | 0.985 | 0.980 | 0.975 | 0.971 | 0.966 | 0.962 | 0.958 | 0.954 | 0.949 | 0.945 | |
| Discharging at 25 °C | 1 | 0.991 | 0.986 | 0.982 | 0.977 | 0.973 | 0.969 | 0.965 | 0.961 | 0.957 | 0.952 | |
| Temperature | 0 °C | 25 °C | |
|---|---|---|---|
| Method | |||
| Measured | 0% | 0% | |
| Nominal | 11.11% | 6.56% | |
| Conventional | 9.05% | 6.38% | |
| Proposed | 2.73% | 1.84% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bašić, H.; Bobanac, V.; Pandžić, H. Determination of Lithium-Ion Battery Capacity for Practical Applications. Batteries 2023, 9, 459. https://doi.org/10.3390/batteries9090459
Bašić H, Bobanac V, Pandžić H. Determination of Lithium-Ion Battery Capacity for Practical Applications. Batteries. 2023; 9(9):459. https://doi.org/10.3390/batteries9090459
Chicago/Turabian StyleBašić, Hrvoje, Vedran Bobanac, and Hrvoje Pandžić. 2023. "Determination of Lithium-Ion Battery Capacity for Practical Applications" Batteries 9, no. 9: 459. https://doi.org/10.3390/batteries9090459