Redox Flow Batteries: Recent Development in Main Components, Emerging Technologies, Diagnostic Techniques, Large-Scale Applications, and Challenges and Barriers
Abstract
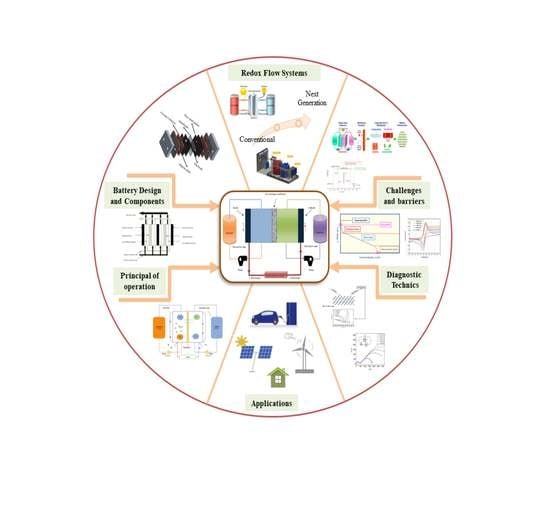
:1. Introduction
2. Overview of Battery Energy Storage Systems Technologies
3. Flow Batteries: Electrochemical Energy Conversion and Storage
4. Components of Redox Flow Batteries
4.1. Membrane
4.1.1. Membrane Development
Ion Exchange Membranes (IEMs)
Porous Membranes
4.1.2. Membrane Evaluation
4.2. Electrode
4.3. Electrolyte
5. Redox Flow Battery Systems
5.1. Commercial Redox Flow Batteries
| Flow Battery | Redox Reaction | Performance | Component | Advantages | Limitation | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cell Voltage | Cycle Efficiency (%) | Energy Efficiency(%) | Electrolyte | Electrode | Commercial Membrane | ||||
| Vanadium | VO2+ + e− ⇌ VO2+ V2+ ⇌ V3+ + e− | ~1.26 | 70–85 | 81–87 | 1.7–2 M V in 4–5 H2SO4 | Thick felt heat bonded graphite impregnated polyethylene plate | Nafion | High energy efficiency, long cycle life, and excellent scalability | Relatively low energy density and high cost of vanadium |
| Fe-Cr | Fe2+ + e− = Fe3+ Cr2+ ⇌ Cr3+ + e − | ~1.81 | 70–85 | 73 | 1 M FeCl2/1 M CrCl3 in 2–3 M HCl | Carbon felt, graphite felt | Nafion | Abundant and low-cost materials, long cycle life | Slow kinetics of Cr2+/Cr3+ reaction. |
| Zn/Br | Br2 + 2e− ⇌ 2Br− Zn ⇌ Zn2+ + 2e− | ~1.85 | 75–80 | 69.4 | ZnBr2 in excess of Br2 (ZnBr2 oil) | Graphite, carbon paper | Nafion, PTFE | High energy density, low cost, long cycle life | Bromine cross-over, complex system management |
5.2. Next-Generation Flow-Batteries
5.2.1. Semi-Solid Redox Flow Batteries
5.2.2. Solid Mediated/Targeted/Boosted Redox Flow Batteries
5.2.3. Solar Redox Flow Batteries
5.2.4. Air-Breathing Sulfur Flow Batteries
5.2.5. Metal–CO2 Batteries

| Systems | Electrode Material | Negative Electrolyte | Positive Electrolyte | Back Ground Electrolyte | Membrane | Charge Voltage (App. Value) (V) | Discharge Voltage (V) | Current Density (mA cm−2) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Cr-EDTA/Cr-EDTA | Graphite felt and rod | 0.05 M Cr (III)-EDTA | 0.1 M Cr (III)-EDTA | 1 M Sodium Acetate | Cation-ex Nafion® 450 | 1.4 (H-cell) | NA | 0.57 (H-cell) | [274,275] |
| Fe-Cr | Negative: carbon fiber Positive: Carbon felt + catalyst | 1M CrCl3 | 1M FeCl2 | 2 M HCl | Cation-ex Nafion® 117 | 1.05 | 1.03 | 21.5 | [276,277] |
| Ti-Fe | Negative: Carbon felt + bismuth (bi) catalyst | Ti (III)/Ti (II) | Fe (III)/Fe (II) | (1–4) M H2SO4 | sulfonated poly (ether ketone) (SPEEK) | NA | 0.4 | 40–120 | [278] |
| All vanadium | Thick felt heat bonded graphite impregnated polyethylene plate | 2 M V (II)/V (III) | 2 M V (IV)/V (V) | 2M H2SO4 | Cation-ex polystyrene sulphonic acid membrane | 1.47 | 1.30 | 30 | [145] |
| Vanadium chloride polyhalide | Graphite felts compressed | 1M VCl3 | 1 M NaBr | 1.5 M HCl | Cation-ex Nafion® 112 membrane | 1.2 | 0.98 | 20 | [192] |
| Ce-V | Carbon fibers | 0.5M Ce (IV)/Ce (III) | 0.5 M V (III)/V (II) | 1 M H2SO4 | Vycor glass membrane (Asahi Glass Co., Ltd.) | 1.83 | 1.51 | 22 | [279] |
6. Diagnostics and Material Characterization Techniques

7. Redox Flow Battery for Large-Scale Energy Storage Application
8. Engineering Aspects
8.1. Reactor Design
8.2. Current Distribution
8.3. Shunt Current
8.4. Gas Evolution
9. Challenges and Barriers
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Skyllas-Kazacos, M.; Menictas, C.; Lim, T. Redox flow batteries for medium-to large-scale energy storage. In Electricity Transmission, Distribution and Storage Systems; Elsevier: Amsterdam, The Netherlands, 2013; pp. 398–441. [Google Scholar]
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M.A. Renewable Energy and Energy Storage Systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Alami, A.H. Introduction to mechanical energy storage. In Mechanical Energy Storage for Renewable and Sustainable Energy Resources; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–12. [Google Scholar]
- Yang, Z.; Du, H.; Jin, L.; Poelman, D. High-performance lead-free bulk ceramics for electrical energy storage applications: Design strategies and challenges. J. Mater. Chem. A 2021, 9, 18026–18085. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelghafar, A.A.; Maghrabie, H.M.; Sayed, E.T.; Rezk, H.; Radi, M.A.; Obaideen, K.; Abdelkareem, M.A. Application of artificial intelligence for prediction, optimization, and control of thermal energy storage systems. Therm. Sci. Eng. Prog. 2023, 39, 101730. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Sayed, E.T. Carbon-Based Materials for Supercapacitors: Recent Progress, Challenges and Barriers. Batteries 2023, 9, 19. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Al-Murisi, M.; Shehata, N.; Alami, A.H.; Radwan, A.; Wilberforce, T.; Chae, K.-J.; Sayed, E.T. Recent progress in Green Ammonia: Production, applications, assessment; barriers, and its role in achieving the sustainable development goals. Energy Convers. Manag. 2023, 277, 116594. [Google Scholar] [CrossRef]
- Permatasari, F.A.; Irham, M.A.; Bisri, S.Z.; Iskandar, F. Carbon-based quantum dots for supercapacitors: Recent advances and future challenges. Nanomaterials 2021, 11, 91. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [Green Version]
- Gandomi, Y.A.; Aaron, D.; Houser, J.; Daugherty, M.; Clement, J.; Pezeshki, A.; Ertugrul, T.; Moseley, D.; Mench, M. Critical review—Experimental diagnostics and material characterization techniques used on redox flow batteries. J. Electrochem. Soc. 2018, 165, A970. [Google Scholar]
- Guney, M.S.; Tepe, Y. Classification and assessment of energy storage systems. Renew. Sustain. Energy Rev. 2017, 75, 1187–1197. [Google Scholar]
- Poullikkas, A. A comparative overview of large-scale battery systems for electricity storage. Renew. Sustain. Energy Rev. 2013, 27, 778–788. [Google Scholar]
- Soloveichik, G. Flow batteries: Current status and trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar]
- Zhao, J.; Burke, A. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar]
- Alnaqbi, H.; El-Kadri, O.; Abdelkareem, M.A.; Al-Asheh, S. Recent Progress in Metal-Organic Framework-Derived Chalcogenides (MX.; X = S, Se) as Electrode Materials for Supercapacitors and Catalysts in Fuel Cells. Energies 2022, 15, 8229. [Google Scholar]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Abo-Khalil, A.G.; Maghrabie, H.M.; Elsaid, K.; Abdelkareem, M.A. Battery energy storage systems and SWOT (strengths, weakness, opportunities, and threats) analysis of batteries in power transmission. Energy 2022, 254, 123987. [Google Scholar] [CrossRef]
- Gupta, N.; Kaur, N.; Jain, S.K.; Joshal, K.S. Chapter 3—Smart grid power system. In Advances in Smart Grid Power System; Tomar, A., Kandari, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 47–71. [Google Scholar]
- Qiao, Y.; Zhao, H.; Shen, Y.; Li, L.; Rao, Z.; Shao, G.; Lei, Y. Recycling of graphite anode from spent lithium-ion batteries: Advances and perspectives. Adv. Perspect. 2023, 5, e12321. [Google Scholar]
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Pérez, G.; Ortiz, A.; Ortiz, I. The roles of ionic liquids as new electrolytes in redox flow batteries. Sep. Purif. Technol. 2020, 252, 117436. [Google Scholar]
- Ye, R.; Henkensmeier, D.; Yoon, S.J.; Huang, Z.; Kim, D.K.; Chang, Z.; Kim, S.; Chen, R. Redox flow batteries for energy storage: A technology review. J. Electrochem. Energy Convers. Storage 2018, 15, 010801. [Google Scholar]
- Leung, P.; Shah, A.; Sanz, L.; Flox, C.; Morante, J.; Xu, Q.; Mohamed, M.; De León, C.P.; Walsh, F. Recent developments in organic redox flow batteries: A critical review. J. Power Sources 2017, 360, 243–283. [Google Scholar]
- Bamgbopa, M.O.; Fetyan, A.; Vagin, M.; Adelodun, A.A. Towards eco-friendly redox flow batteries with all bio-sourced cell components. J. Energy Storage 2022, 50, 104352. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar]
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The chemistry of redox-flow batteries. Angew. Chem. Int. Ed. 2015, 54, 9776–9809. [Google Scholar]
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U. Redox-flow batteries: From metals to organic redox-active materials. Angew. Chem. Int. Ed. 2017, 56, 686–711. [Google Scholar]
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox flow batteries: Status and perspective towards sustainable stationary energy storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- May, G.J.; Davidson, A.; Monahov, B.J. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar]
- Hirsch, A.; Parag, Y.; Guerrero, J. Microgrids: A review of technologies, key drivers, and outstanding issues. Renew. Sustain. Energy Rev. 2018, 90, 402–411. [Google Scholar]
- Das, C.K.; Bass, O.; Kothapalli, G.; Mahmoud, T.S.; Habibi, D.; Reviews, S.E. Overview of energy storage systems in distribution networks: Placement, sizing, operation, and power quality. Renew. Sustain. Energy Rev. 2018, 91, 1205–1230. [Google Scholar]
- Weil, M.; Peters, J.F.; Chibeles-Martins, N.; Moniz, A.B.; Reviews, S.E. A review of multi-criteria decision making approaches for evaluating energy storage systems for grid applications. Renew. Sustain. Energy Rev. Baumann Man. 2019, 107, 516–534. [Google Scholar]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar]
- Wang, Z.; Wang, Z.; Peng, W.; Guo, H.; Li, X.; Wang, J.; Qi, A. Structure and electrochemical performance of LiCoO2 cathode material in different voltage ranges. Ionics 2014, 20, 1525–1534. [Google Scholar]
- Zheng, H.; Wang, T.; Zhao, R.; Chen, J.; Li, L. LiMn2O4 microspheres as high-performance cathode materials for Li-ion batteries. In IOP Conference Series: Earth and Environmental Science; No. 2; IOP Publishing: Tokyo, Japan, 2018; Volume 108, p. 022011. [Google Scholar]
- Du, W.; Xue, N.; Sastry, A.M.; Martins, J.R.; Shyy, W. Energy density comparison of Li-ion cathode materials using dimensional analysis. J. Electrochem. Soc. 2013, 160, A1187. [Google Scholar]
- Hannan, M.A.; Wali, S.B.; Ker, P.J.; Rahman, M.S.A.; Mansor, M.; Ramachandaramurthy, V.K.; Dong, Z.Y. Battery energy-storage system: A review of technologies, optimization objectives, constraints, approaches, and outstanding issues. J. Energy Storage 2021, 42, 103023. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I.J.E. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar]
- Liang, Y.; Zhao, C.-Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.-Q.; Yu, D.; Liu, Y.; Titirici, M.-M.; Chueh, Y.-L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef] [Green Version]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G.J.R.; Reviews, S.E. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar]
- Nazir, A.; Le, H.T.; Nguyen, A.-G.; Park, C.-J. Graphene analogue metal organic framework with superior capacity and rate capability as an anode for lithium ion batteries. Electrochim. Acta 2021, 389, 138750. [Google Scholar]
- Nayem, S.A.; Ahmad, A.; Shah, S.S.; Alzahrani, A.S.; Ahammad, A.S.; Aziz, M. High Performance and Long-cycle Life Rechargeable Aluminum Ion Battery: Recent Progress, Perspectives and Challenges. Chem. Rec. 2022, 22, e202200181. [Google Scholar]
- Li, M.; Ding, Y.; Sun, Y.; Ren, Y.; Yang, J.; Yin, B.; Li, H.; Zhang, S.; Ma, T. Emerging rechargeable aqueous magnesium ion battery. Mater. Rep. Energy 2022, 2, 100161. [Google Scholar] [CrossRef]
- Zampardi, G.; Mantia, F.L. Open challenges and good experimental practices in the research field of aqueous Zn-ion batteries. Nat. Commun. 2022, 13, 687. [Google Scholar] [CrossRef]
- Nazir, A.; Le, H.T.T.; Nguyen, A.-G.; Kim, J.; Park, C.-J. Conductive metal organic framework mediated Sb nanoparticles as high-capacity anodes for rechargeable potassium-ion batteries. Chem. Eng. J. 2022, 450, 138408. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Prakash, A. Advancement of technology towards developing Na-ion batteries. J. Power Sources 2018, 378, 268–300. [Google Scholar]
- Robb, B.H.; Waters, S.E.; Marshak, M.P. Evaluating aqueous flow battery electrolytes: A coordinated approach. Dalton Trans. 2020, 49, 16047–16053. [Google Scholar] [PubMed]
- Huang, Y.; Gu, S.; Yan, Y.; Li, S. Nonaqueous redox-flow batteries: Features, challenges, and prospects. Curr. Opin. Chem. Eng. 2015, 8, 105–113. [Google Scholar]
- Kowalski, J.A.; Su, L.; Milshtein, J.D.; Brushett, F.R. Recent advances in molecular engineering of redox active organic molecules for nonaqueous flow batteries. Curr. Opin. Chem. Eng. 2016, 13, 45–52. [Google Scholar]
- Hogue, R.W.; Toghill, K.E. Metal coordination complexes in nonaqueous redox flow batteries. Curr. Opin. Electrochem. 2019, 18, 37–45. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, R.; Ma, Q.; Li, Z.; Su, H.; Lu, P.; Yang, W.; Xu, Q. Modeling and Simulation of Non-Aqueous Redox Flow Batteries: A Mini-Review. Batteries 2023, 9, 215. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Yao, Y.; Wang, W.; Lu, Y.-C. High-areal-capacity conversion type iron-based hybrid redox flow batteries. Energy Environ. Sci. 2021, 14, 6329–6337. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, Q.; Li, Y.; Wang, J.; Lund, P.D. Review of zinc dendrite formation in zinc bromine redox flow battery. Renew. Sustain. Energy Rev. 2020, 127, 109838. [Google Scholar] [CrossRef]
- Zhang, L.; Lai, Q.; Zhang, J.; Zhang, H. A high-energy-density redox flow battery based on zinc/polyhalide chemistry. ChemSusChem 2012, 5, 867–869. [Google Scholar]
- Mohamed, M.R.; Sharkh, S.M.; Walsh, F.C. Redox flow batteries for hybrid electric vehicles: Progress and challenges. In Proceedings of the 2009 IEEE Vehicle Power and Propulsion Conference, Dearborn, MI, USA, 7–10 September 2009; pp. 551–557. [Google Scholar]
- Palanisamy, G.; Oh, T.H. TiO2 Containing Hybrid Composite Polymer Membranes for Vanadium Redox Flow Batteries. Polymers 2022, 14, 1617. [Google Scholar] [CrossRef]
- Darling, R.M.; Weber, A.Z.; Tucker, M.C.; Perry, M.L. The Influence of Electric Field on Crossover in Redox-Flow Batteries. J. Electrochem. Soc. 2016, 163, A5014. [Google Scholar] [CrossRef]
- Sawant, T.V.; Yim, C.S.; Henry, T.J.; Miller, D.M.; McKone, J.R. Harnessing Interfacial Electron Transfer in Redox Flow Batteries. Joule 2021, 5, 360–378. [Google Scholar] [CrossRef]
- Izquierdo-Gil, M.A.; Villaluenga, J.P.G.; Muñoz, S.; Barragán, V.M. The Correlation between the Water Content and Electrolyte Permeability of Cation-Exchange Membranes. Int. J. Mol. Sci. 2020, 21, 5897. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar]
- Xia, Y.; Ouyang, M.; Yufit, V.; Tan, R.; Regoutz, A.; Wang, A.; Mao, W.; Chakrabarti, B.; Kavei, A.; Song, Q. A cost-effective alkaline polysulfide-air redox flow battery enabled by a dual-membrane cell architecture. Nat. Commun. 2022, 13, 2388. [Google Scholar]
- Machado, C.A.; Brown, G.O.; Yang, R.; Hopkins, T.E.; Pribyl, J.G.; Epps, T.H., III. Redox Flow Battery Membranes: Improving Battery Performance by Leveraging Structure–Property Relationships. ACS Energy Lett. 2021, 6, 158–176. [Google Scholar] [CrossRef]
- Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T.M.; Ukil, A.; Zhao, J. Recent development of membrane for vanadium redox flow battery applications: A review. Appl. Energy 2019, 238, 202–224. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Liu, J.; Zhou, Q.; Zhou, W.; Li, H.; Wu, Y. Ion exchange membranes for vanadium redox flow batteries. Pure Appl. Chem. 2014, 86, 633–649. [Google Scholar] [CrossRef]
- Elgammal, R.A.; Tang, Z.; Sun, C.-N.; Lawton, J.; Zawodzinski, T.A. Species Uptake and Mass Transport in Membranes for Vanadium Redox Flow Batteries. Electrochim. Acta 2017, 237, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Nie, Z.; Luo, Q.; Li, B.; Chen, B.; Simmons, K.; Sprenkle, V.; Wang, W. Nanoporous polytetrafluoroethylene/silica composite separator as a high-performance all-vanadium redox flow battery membrane. Adv. Energy Mater. 2013, 3, 1215–1220. [Google Scholar]
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Thiam, B.G.; Vaudreuil, S. Recent membranes for vanadium redox flow batteries. J. Electrochem. Soc. 2021, 168, 070553. [Google Scholar] [CrossRef]
- Düerkop, D.; Widdecke, H.; Schilde, C.; Kunz, U.; Schmiemann, A. Polymer Membranes for All-Vanadium Redox Flow Batteries: A Review. Membranes 2021, 11, 214. [Google Scholar] [PubMed]
- Xi, J.; Wu, Z.; Teng, X.; Zhao, Y.; Chen, L.; Qiu, X. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries. J. Mater. Chem. 2008, 18, 1232–1238. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, M.G.; Roh, S.-H.; Jung, H.-Y. Recent developments in sol-gel based polymer electrolyte membranes for vanadium redox flow batteries—A review. Polym. Test. 2020, 89, 106567. [Google Scholar] [CrossRef]
- Jung, M.; Lee, W.; Nambi Krishnan, N.; Kim, S.; Gupta, G.; Komsiyska, L.; Harms, C.; Kwon, Y.; Henkensmeier, D. Porous-Nafion/PBI composite membranes and Nafion/PBI blend membranes for vanadium redox flow batteries. Appl. Surf. Sci. 2018, 450, 301–311. [Google Scholar] [CrossRef]
- Cho, H.; Krieg, H.M.; Kerres, J.A. Performances of Anion-Exchange Blend Membranes on Vanadium Redox Flow Batteries. Membranes 2019, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Jung, M.; Serhiichuk, D.; Noh, C.; Gupta, G.; Harms, C.; Kwon, Y.; Henkensmeier, D. Layered composite membranes based on porous PVDF coated with a thin, dense PBI layer for vanadium redox flow batteries. J. Membr. Sci. 2019, 591, 117333. [Google Scholar] [CrossRef]
- Su, L.; Zhang, D.; Peng, S.; Wu, X.; Luo, Y.; He, G. Orientated graphene oxide/Nafion ultra-thin layer coated composite membranes for vanadium redox flow battery. Int. J. Hydrog. Energy 2017, 42, 21806–21816. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Dhanabalan, K.; Thong, P.T.; Kim, J.Y.; Roh, S.H.; Jung, H. Development of perfluorosulfonic acid polymer-based hybrid composite membrane with alkoxysilane functionalized polymer for vanadium redox flow battery. Int. J. Energy Res. 2020, 44, 1999–2010. [Google Scholar] [CrossRef]
- Thong, P.T.; Sadhasivam, T.; Lim, H.; Jin, C.-S.; Ryi, S.-K.; Park, W.; Kim, H.T.; Roh, S.-H.; Jung, H.-Y. High oxidizing stability and ion selectivity of hybrid polymer electrolyte membrane for improving electrochemical performance in vanadium redox flow battery. J. Electrochem. Soc. 2018, 165, A2321. [Google Scholar] [CrossRef]
- Palanisamy, G.; Sadhasivam, T.; Park, W.-S.; Bae, S.T.; Roh, S.-H.; Jung, H.-Y. Tuning the ion selectivity and chemical stability of a biocellulose membrane by PFSA ionomer reinforcement for vanadium redox flow battery applications. ACS Sustain. Chem. Eng. 2020, 8, 2040–2051. [Google Scholar] [CrossRef]
- Mu, D.; Yu, L.; Liu, L.; Xi, J. Rice paper reinforced sulfonated poly (ether ether ketone) as low-cost membrane for vanadium flow batteries. ACS Sustain. Chem. Eng. 2017, 5, 2437–2444. [Google Scholar]
- Cui, Y.; Chen, X.; Wang, Y.; Peng, J.; Zhao, L.; Du, J.; Zhai, M. Amphoteric Ion Exchange Membranes Prepared by Preirradiation-Induced Emulsion Graft Copolymerization for Vanadium Redox Flow Battery. Polymers 2019, 11, 1482. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, M.; Kumar, S.; Singh, A.K.; Shahi, V.K. High-performance membrane for vanadium redox flow batteries: Cross-linked poly (ether ether ketone) grafted with sulfonic acid groups via the spacer. J. Membr. Sci. 2019, 583, 1–8. [Google Scholar] [CrossRef]
- Charyton, M.; Deboli, F.; Fischer, P.; Henrion, G.; Etienne, M.; Donten, M.L. Composite Anion Exchange Membranes Fabricated by Coating and UV Crosslinking of Low-Cost Precursors Tested in a Redox Flow Battery. Polymers 2021, 13, 2396. [Google Scholar]
- Zeng, L.; Zhao, T.S.; Wei, L.; Zeng, Y.K.; Zhang, Z.H. Highly stable pyridinium-functionalized cross-linked anion exchange membranes for all vanadium redox flow batteries. J. Power Sources 2016, 331, 452–461. [Google Scholar] [CrossRef]
- Wang, L.; Yu, L.; Mu, D.; Yu, L.; Wang, L.; Xi, J. Acid-base membranes of imidazole-based sulfonated polyimides for vanadium flow batteries. J. Membr. Sci. 2018, 552, 167–176. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; He, Z.; Das, R.; Dong, B.; Xie, X.; Guo, Z. An overview of amphoteric ion exchange membranes for vanadium redox flow batteries. J. Mater. Sci. Technol. 2021, 69, 212–227. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yang, M.-C.; Wei, H.-J. Amino-silica modified Nafion membrane for vanadium redox flow battery. J. Power Sources 2015, 282, 562–571. [Google Scholar] [CrossRef]
- Lou, X.; Yuan, D.; Yu, Y.; Lei, Y.; Ding, M.; Sun, Q.; Jia, C. A Cost-effective Nafion Composite Membrane as an Effective Vanadium-Ion Barrier for Vanadium Redox Flow Batteries. Chem.-Asian J. 2020, 15, 2357–2363. [Google Scholar] [PubMed]
- Ye, J.; Yuan, D.; Ding, M.; Long, Y.; Long, T.; Sun, L.; Jia, C. A cost-effective nafion/lignin composite membrane with low vanadium ion permeation for high performance vanadium redox flow battery. J. Power Sources 2021, 482, 229023. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, H.; Chen, J.; Qian, P.; Zhai, Y. Modification of Nafion membrane using interfacial polymerization for vanadium redox flow battery applications. J. Membr. Sci. 2008, 311, 98–103. [Google Scholar] [CrossRef]
- An, H.; Zhang, R.; Li, W.; Li, P.; Qian, H.; Yang, H. Surface-Modified Approach to Fabricate Nafion Membranes Covalently Bonded with Polyhedral Oligosilsesquioxane for Vanadium Redox Flow Batteries. ACS Appl. Mater. Interfaces 2022, 14, 7845–7855. [Google Scholar] [CrossRef]
- Jiang, B.; Yu, L.; Wu, L.; Mu, D.; Liu, L.; Xi, J.; Qiu, X. Insights into the Impact of the Nafion Membrane Pretreatment Process on Vanadium Flow Battery Performance. ACS Appl. Mater. Interfaces 2016, 8, 12228–12238. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, L.-H.; Kwon, Y.; Kim, S. Effect of nafion membrane thickness on performance of vanadium redox flow battery. Korean J. Chem. Eng. 2014, 31, 2081–2087. [Google Scholar] [CrossRef]
- Thong, P.T.; Ajeya, K.V.; Dhanabalan, K.; Roh, S.-H.; Son, W.-K.; Park, S.-C.; Jung, H.-Y. A coupled-layer ion-conducting membrane using composite ionomer and porous substrate for application to vanadium redox flow battery. J. Power Sources 2022, 521, 230912. [Google Scholar] [CrossRef]
- Abdiani, M.; Abouzari-Lotf, E.; Ting, T.M.; Moozarm Nia, P.; Sha’rani, S.S.; Shockravi, A.; Ahmad, A. Novel polyolefin based alkaline polymer electrolyte membrane for vanadium redox flow batteries. J. Power Sources 2019, 424, 245–253. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, D.; Zhao, L.; Wang, S.; Liu, J.; Yan, C. Excellent ion selectivity of Nafion membrane modified by PBI via acid-base pair effect for vanadium flow battery. Electrochim. Acta 2021, 394, 139144. [Google Scholar] [CrossRef]
- Jia, C.; Liu, J.; Yan, C. A multilayered membrane for vanadium redox flow battery. J. Power Sources 2012, 203, 190–194. [Google Scholar] [CrossRef]
- Teng, X.; Dai, J.; Su, J.; Zhu, Y.; Liu, H.; Song, Z. A high performance polytetrafluoroethene/Nafion composite membrane for vanadium redox flow battery application. J. Power Sources 2013, 240, 131–139. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, C.; Liu, J.; Sun, T.; Yu, S.; Li, H. In Situ Grown Tungsten Trioxide Nanoparticles on Graphene Oxide Nanosheet to Regulate Ion Selectivity of Membrane for High Performance Vanadium Redox Flow Battery. Adv. Funct. Mater. 2021, 32, 2109427. [Google Scholar] [CrossRef]
- Assink, R. Fouling mechanism of separator membranes for the iron/chromium redox battery. J. Membr. Sci. 1984, 17, 205–217. [Google Scholar] [CrossRef]
- Ling, J.S.; Charleston, J. Advances in Membrane Technology for the NASA Redox Energy-Storage System; NASA Lewis Research Center: Cleveland, OH, USA, 1980. [Google Scholar]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Selective anion exchange membranes for high coulombic efficiency vanadium redox flow batteries. Electrochem. Commun. 2013, 26, 37–40. [Google Scholar] [CrossRef]
- Wandschneider, F.T.; Finke, D.; Grosjean, S.; Fischer, P.; Pinkwart, K.; Tübke, J.; Nirschl, H. Model of a vanadium redox flow battery with an anion exchange membrane and a Larminie-correction. J. Power Sources 2014, 272, 436–447. [Google Scholar] [CrossRef]
- Delgado, N.M.; Monteiro, R.; Abdollahzadeh, M.; Ribeirinha, P.; Bentien, A.; Mendes, A. 2D-dynamic phenomenological modelling of vanadium redox flow batteries—Analysis of the mass transport related overpotentials. J. Power Sources 2020, 480, 229142. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Tuning poly (arylene piperidinium) anion-exchange membranes by copolymerization, partial quaternization and crosslinking. J. Membr. Sci. 2019, 578, 183–195. [Google Scholar] [CrossRef]
- Li, N.; Cui, Z.; Zhang, S.; Li, S.; Zhang, F. Preparation and evaluation of a proton exchange membrane based on oxidation and water stable sulfonated polyimides. J. Power Sources 2007, 172, 511–519. [Google Scholar] [CrossRef]
- Son, T.Y.; Kim, D.J.; Vijayakumar, V.; Kim, K.; Kim, D.S.; Nam, S.Y. Anion exchange membrane using poly(ether ether ketone) containing imidazolium for anion exchange membrane fuel cell (AEMFC). J. Ind. Eng. Chem. 2020, 89, 175–182. [Google Scholar] [CrossRef]
- Li, S.; Zhu, X.; Liu, D.; Sun, F. A highly durable long side-chain polybenzimidazole anion exchange membrane for AEMFC. J. Membr. Sci. 2018, 546, 15–21. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lim, D.-H.; Chae, J.E.; Choi, J.; Kim, B.H.; Lee, S.Y.; Yoon, C.W.; Nam, S.Y.; Jang, J.H.; Henkensmeier, D.; et al. Base tolerant polybenzimidazolium hydroxide membranes for solid alkaline-exchange membrane fuel cells. J. Membr. Sci. 2016, 514, 398–406. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Ryu, C.Y.; Kim, Y.S.; Bae, C. Stable Elastomeric Anion Exchange Membranes Based on Quaternary Ammonium-Tethered Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers. Macromolecules 2015, 48, 7085–7095. [Google Scholar] [CrossRef]
- Son, T.Y.; Choi, D.H.; Park, C.H.; Nam, S.Y. Preparation and electrochemical characterization of membranes using submicron sized particles with high ion exchange capacity for electro-adsorptive deionization. J. Nanosci. Nanotechnol. 2017, 17, 7743–7750. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Park, S.; Han, J.; Maurya, S.; Mohanty, A.D.; Tian, D.; Saikia, N.; Hickner, M.A.; Ryu, C.Y.; Tuckerman, M.E.; et al. Synthesis of Aromatic Anion Exchange Membranes by Friedel–Crafts Bromoalkylation and Cross-Linking of Polystyrene Block Copolymers. Macromolecules 2019, 52, 2139–2147. [Google Scholar] [CrossRef]
- Son, T.Y.; Kim, T.-H.; Nam, S.Y. Crosslinked Pore-Filling Anion Exchange Membrane Using the Cylindrical Centrifugal Force for Anion Exchange Membrane Fuel Cell System. Polymers 2020, 12, 2758. [Google Scholar]
- Son, T.Y.; Im, K.S.; Jung, H.N.; Nam, S.Y. Blended Anion Exchange Membranes for Vanadium Redox Flow Batteries. Polymers 2021, 13, 2827. [Google Scholar]
- Cho, H.; Krieg, H.M.; Kerres, J.A. Application of Novel Anion-Exchange Blend Membranes (AEBMs) to Vanadium Redox Flow Batteries. Membranes 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Zhang, H.; Li, X. Ion conducting membranes for aqueous flow battery systems. Chem. Commun. 2018, 54, 7570–7588. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, C.; Zhen, Y.; Zhao, Y.; Li, Y. Enhancing the performance of an all-organic non-aqueous redox flow battery. J. Power Sources 2019, 443, 227283. [Google Scholar] [CrossRef]
- Yuan, J.; Xia, Y.; Chen, X.; Zhao, Y.; Li, Y. Recent development in two-dimensional material-based membranes for redox flow battery. Curr. Opin. Chem. Eng. 2022, 38, 100856. [Google Scholar] [CrossRef]
- Zheng, Z.-J.; Ye, H.; Guo, Z.-P. Recent progress on pristine metal/covalent-organic frameworks and their composites for lithium–sulfur batteries. Energy Environ. Sci. 2021, 14, 1835–1853. [Google Scholar] [CrossRef]
- Miró, P.; Audiffred, M.; Heine, T. An atlas of two-dimensional materials. Chem. Soc. Rev. 2014, 43, 6537–6554. [Google Scholar] [CrossRef]
- Chen, Q.; Du, Y.-Y.; Li, K.-M.; Xiao, H.-F.; Wang, W.; Zhang, W.-M. Graphene enhances the proton selectivity of porous membrane in vanadium flow batteries. Mater. Des. 2017, 113, 149–156. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Zhang, C.; Zhou, Y.; Zhou, X.; Liu, Z.; Yu, G. Enabling Graphene-Oxide-Based Membranes for Large-Scale Energy Storage by Controlling Hydrophilic Microstructures. Chem 2018, 4, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Di, M.; Xiu, Y.; Dong, Z.; Hu, L.; Gao, L.; Dai, Y.; Yan, X.; Zhang, N.; Pan, Y.; Jiang, X.; et al. Two-dimensional MoS2 nanosheets constructing highly ion-selective composite membrane for vanadium redox flow battery. J. Membr. Sci. 2021, 623, 119051. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Ban, Y.; Yang, W. Two-dimensional metal–organic framework nanosheets for membrane-based gas separation. Angew. Chem. 2017, 129, 9889–9893. [Google Scholar] [CrossRef]
- Tian, W.; Li, Z.; Ge, Z.; Xu, D.; Zhang, K. Self-assembly of vermiculite-polymer composite films with improved mechanical and gas barrier properties. Appl. Clay Sci. 2019, 180, 105198. [Google Scholar] [CrossRef]
- Dai, W.; Shen, Y.; Li, Z.; Yu, L.; Xi, J.; Qiu, X. SPEEK/Graphene oxide nanocomposite membranes with superior cyclability for highly efficient vanadium redox flow battery. J. Mater. Chem. A 2014, 2, 12423–12432. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, Y.; Yang, P.; Yang, H.; Xuan, S.; Long, J.; Wang, Y.; Zhang, H. Branched sulfonated polyimide/functionalized silicon carbide composite membranes with improved chemical stabilities and proton selectivities for vanadium redox flow battery application. J. Mater. Sci. 2018, 53, 14506–14524. [Google Scholar] [CrossRef]
- Pu, Y.; Zhu, S.; Wang, P.; Zhou, Y.; Yang, P.; Xuan, S.; Zhang, Y.; Zhang, H. Novel branched sulfonated polyimide/molybdenum disulfide nanosheets composite membrane for vanadium redox flow battery application. Appl. Surf. Sci. 2018, 448, 186–202. [Google Scholar] [CrossRef]
- Wu, C.; Bai, H.; Lv, Y.; Lv, Z.; Xiang, Y.; Lu, S. Enhanced membrane ion selectivity by incorporating graphene oxide nanosheet for vanadium redox flow battery application. Electrochim. Acta 2017, 248, 454–461. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, L.; Yu, L.; Qiu, X.; Xi, J. A comparative study of Nafion series membranes for vanadium redox flow batteries. J. Membr. Sci. 2016, 510, 18–26. [Google Scholar] [CrossRef]
- Reed, D.; Thomsen, E.; Wang, W.; Nie, Z.; Li, B.; Wei, X.; Koeppel, B.; Sprenkle, V. Performance of Nafion® N115, Nafion® NR-212, and Nafion® NR-211 in a 1 kW class all vanadium mixed acid redox flow battery. J. Power Sources 2015, 285, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, T.; Kazacos, M. Evaluation of the chemical stability of some membranes in vanadium solution. J. Appl. Electrochem. 1997, 27, 153–160. [Google Scholar] [CrossRef]
- Sukkar, T.; Skyllas-Kazacos, M. Membrane stability studies for vanadium redox cell applications. J. Appl. Electrochem. 2004, 34, 137–145. [Google Scholar] [CrossRef]
- Cao, L.; Kronander, A.; Tang, A.; Wang, D.-W.; Skyllas-Kazacos, M. Membrane permeability rates of vanadium ions and their effects on temperature variation in vanadium redox batteries. Energies 2016, 9, 1058. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.D.; Whitehead, A.; Wai, N.; Ong, S.J.H.; Scherer, G.G.; Xu, Z.J. Equilibrium and dynamic absorption of electrolyte species in cation/anion exchange membranes of vanadium redox flow batteries. ChemSusChem 2019, 12, 1076–1083. [Google Scholar] [CrossRef]
- Zhao, N.; Riley, H.; Song, C.; Jiang, Z.; Tsay, K.-C.; Neagu, R.; Shi, Z. Ex-Situ Evaluation of Commercial Polymer Membranes for Vanadium Redox Flow Batteries (VRFBs). Polymers 2021, 13, 926. [Google Scholar] [CrossRef]
- Ruiyong, C.; Sangwon, K.; Zhenjun, C. Redox Flow Batteries: Fundamentals and Applications. In Redox; Mohammed, A.A.K., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 5. [Google Scholar]
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium redox flow batteries: A comprehensive review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Couper, A.M.; Pletcher, D.; Walsh, F.C. Electrode materials for electrosynthesis. Chem. Rev. 1990, 90, 837–865. [Google Scholar] [CrossRef]
- De Leon, C.P.; Frías-Ferrer, A.; González-García, J.; Szánto, D.; Walsh, F.C. Redox flow cells for energy conversion. J. Power Sources 2006, 160, 716–732. [Google Scholar] [CrossRef] [Green Version]
- Skyllas-Kazacos, M.; Chakrabarti, M.; Hajimolana, S.; Mjalli, F.; Saleem, M. Progress in flow battery research and development. J. Electrochem. Soc. 2011, 158, R55. [Google Scholar] [CrossRef]
- Bartolozzi, M. Development of redox flow batteries. A historical bibliography. J. Power Sources 1989, 27, 219–234. [Google Scholar] [CrossRef]
- Sum, E.; Skyllas-Kazacos, M. A study of the V (II)/V (III) redox couple for redox flow cell applications. J. Power Sources 1985, 15, 179–190. [Google Scholar] [CrossRef]
- Zhong, S.; Skyllas-Kazacos, M. Electrochemical behaviour of vanadium (V)/vanadium (IV) redox couple at graphite electrodes. J. Power Sources 1992, 39, 1–9. [Google Scholar] [CrossRef]
- Zhong, S.; Kazacos, M.; Burford, R.; Skyllas-Kazacos, M. Fabrication and activation studies of conducting plastic composite electrodes for redox cells. J. Power Sources 1991, 36, 29–43. [Google Scholar] [CrossRef]
- Kazacos, M.; Skyllas-Kazacos, M. Performance Characteristics of Carbon Plastic Electrodes in the All-Vanadium Redox Cell. J. Electrochem. Soc. 1989, 136, 2759. [Google Scholar] [CrossRef]
- Haddadi-Asl, V.; Kazacos, M.; Skyllas-Kazacos, M. Conductive carbon-polypropylene composite electrodes for vanadium redox battery. J. Appl. Electrochem. 1995, 25, 29–33. [Google Scholar] [CrossRef]
- Haddadi-Asl, V.; Kazacos, M.; Skyllas-Kazacos, M. Carbon–polymer composite electrodes for redox cells. J. Appl. Polym. Sci. 1995, 57, 1455–1463. [Google Scholar] [CrossRef]
- Radford, G.; Cox, J.; Wills, R.; Walsh, F. Electrochemical characterisation of activated carbon particles used in redox flow battery electrodes. J. Power Sources 2008, 185, 1499–1504. [Google Scholar] [CrossRef]
- Inoue, M.; Tsuzuki, Y.; Iizuka, Y.; Shimada, M. Carbon fiber electrode for redox flow battery. J. Electrochem. Soc. 1987, 134, 756–757. [Google Scholar] [CrossRef]
- Kaneko, H.; Nozaki, K.; Wada, Y.; Aoki, T.; Negishi, A.; Kamimoto, M. Vanadium redox reactions and carbon electrodes for vanadium redox flow battery. Electrochim. Acta 1991, 36, 1191–1196. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar] [CrossRef]
- Mohammadi, F.; Timbrell, P.; Zhong, S.; Padeste, C.; Skyllas-Kazacos, M. Overcharge in the vanadium redox battery and changes in electrical resistivity and surface functionality of graphite-felt electrodes. J. Power Sources 1994, 52, 61–68. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, X.; Engelhard, M.; Wang, C.; Dai, S.; Liu, J.; Yang, Z.; Lin, Y. Nitrogen-doped mesoporous carbon for energy storage in vanadium redox flow batteries. J. Power Sources 2010, 195, 4375–4379. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazacos, M. Chemical modification of graphite electrode materials for vanadium redox flow battery application—Part II. Acid treatments. Electrochim. Acta 1992, 37, 2459–2465. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazacos, M. Modification of graphite electrode materials for vanadium redox flow battery application—I. Thermal treatment. Electrochim. Acta 1992, 37, 1253–1260. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazakos, M. Chemical modification and electrochemical behaviour of graphite fibre in acidic vanadium solution. Electrochim. Acta 1991, 36, 513–517. [Google Scholar] [CrossRef]
- Rychcik, M.; Skyllas-Kazacos, M. Evaluation of electrode materials for vanadium redox cell. J. Power Sources 1987, 19, 45–54. [Google Scholar] [CrossRef]
- Zhong, S.; Padeste, C.; Kazacos, M.; Skyllas-Kazacos, M. Comparison of the physical, chemical and electrochemical properties of rayon-and polyacrylonitrile-based graphite felt electrodes. J. Power Sources 1993, 45, 29–41. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, M.-S.; Kim, Y.-J.; Kim, J.H.; Dou, S.X.; Skyllas-Kazacos, M. A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries. J. Mater. Chem. A 2015, 3, 16913–16933. [Google Scholar] [CrossRef]
- Iwakiri, I.; Antunes, T.; Almeida, H.; Sousa, J.P.; Figueira, R.B.; Mendes, A.J.E. Redox flow batteries: Materials, design and prospects. Energies 2021, 14, 5643. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Rychcik, M.; Robins, R.G.; Fane, A.; Green, M. New all-vanadium redox flow cell. J. Electrochem. Soc. 1986, 133, 1057. [Google Scholar] [CrossRef]
- Li, X.-G.; Huang, K.-L.; Liu, S.-Q.; Tan, N.; Chen, L.-Q. Characteristics of graphite felt electrode electrochemically oxidized for vanadium redox battery application. Trans. Nonferrous Met. Soc. China 2007, 17, 195–199. [Google Scholar] [CrossRef]
- Wu, T.; Huang, K.; Liu, S.; Zhuang, S.; Fang, D.; Li, S.; Lu, D.; Su, A. Hydrothermal ammoniated treatment of PAN-graphite felt for vanadium redox flow battery. J. Solid State Electrochem. 2012, 16, 579–585. [Google Scholar] [CrossRef]
- González, Z.; Sánchez, A.; Blanco, C.; Granda, M.; Menéndez, R.; Santamaría, R. Enhanced performance of a Bi-modified graphite felt as the positive electrode of a vanadium redox flow battery. Electrochem. Commun. 2011, 13, 1379–1382. [Google Scholar] [CrossRef] [Green Version]
- Park, J.J.; Park, J.H.; Park, O.O.; Yang, J.H. Highly porous graphenated graphite felt electrodes with catalytic defects for high-performance vanadium redox flow batteries produced via NiO/Ni redox reactions. Carbon 2016, 110, 17–26. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Chen, Z.; Waje, M.; Yan, Y. Durability investigation of carbon nanotube as catalyst support for proton exchange membrane fuel cell. J. Power Sources 2006, 158, 154–159. [Google Scholar] [CrossRef]
- Jha, N.; Reddy, A.L.M.; Shaijumon, M.; Rajalakshmi, N.; Ramaprabhu, S. Pt–Ru/multi-walled carbon nanotubes as electrocatalysts for direct methanol fuel cell. Int. J. Hydrog. Energy 2008, 33, 427–433. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Rajalakshmi, N.; Ramaprabhu, S. Cobalt-polypyrrole-multiwalled carbon nanotube catalysts for hydrogen and alcohol fuel cells. Carbon 2008, 46, 2–11. [Google Scholar] [CrossRef]
- Saha, M.S.; Li, R.; Sun, X. High loading and monodispersed Pt nanoparticles on multiwalled carbon nanotubes for high performance proton exchange membrane fuel cells. J. Power Sources 2008, 177, 314–322. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. Multi-walled carbon nanotubes used as an electrode reaction catalyst for VO2+/VO2+ for a vanadium redox flow battery. Carbon 2011, 49, 3463–3470. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. Graphite–graphite oxide composite electrode for vanadium redox flow battery. Electrochim. Acta 2011, 56, 5290–5294. [Google Scholar] [CrossRef]
- Stauber, J.M.; Zhang, S.; Gvozdik, N.; Jiang, Y.; Avena, L.; Stevenson, K.J.; Cummins, C.C. Cobalt and vanadium trimetaphosphate polyanions: Synthesis, characterization, and electrochemical evaluation for non-aqueous redox-flow battery applications. J. Am. Chem. Soc. 2018, 140, 538–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, A.; Wai, N.; Schweiss, R.; Whitehead, A.; Lim, T.M.; Hng, H.H. Advanced porous electrodes with flow channels for vanadium redox flow battery. J. Power Sources 2017, 341, 83–90. [Google Scholar] [CrossRef]
- Houser, J.; Pezeshki, A.; Clement, J.T.; Aaron, D.; Mench, M.M. Architecture for improved mass transport and system performance in redox flow batteries. J. Power Sources 2017, 351, 96–105. [Google Scholar] [CrossRef]
- Leung, P.; Ponce-de-León, C.; Low, C.; Shah, A.; Walsh, F. Characterization of a zinc–cerium flow battery. J. Power Sources 2011, 196, 5174–5185. [Google Scholar] [CrossRef]
- Nikiforidis, G.; Berlouis, L.; Hall, D.; Hodgson, D. Evaluation of carbon composite materials for the negative electrode in the zinc–cerium redox flow cell. J. Power Sources 2012, 206, 497–503. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Grossmith, F. Efficient vanadium redox flow cell. J. Electrochem. Soc. 1987, 134, 2950. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, H.; Zhao, P.; Yi, B. A comparative study of carbon felt and activated carbon based electrodes for sodium polysulfide/bromine redox flow battery. Electrochim. Acta 2006, 51, 6304–6312. [Google Scholar] [CrossRef]
- Pletcher, D.; Wills, R. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead (II) Part II. Flow cell studies. Phys. Chem. Chem. Phys. 2004, 6, 1779–1785. [Google Scholar] [CrossRef]
- Hagg, C.M.; Skyllas-Kazacos, M. Novel bipolar electrodes for battery applications. J. Appl. Electrochem. 2002, 32, 1063–1069. [Google Scholar] [CrossRef]
- Jorné, J.; Kim, J.; Kralik, D. The zinc-chlorine battery: Half-cell overpotential measurements. J. Appl. Electrochem. 1979, 9, 573–579. [Google Scholar] [CrossRef]
- Kinoshita, K.; Leach, S. Mass-transfer study of carbon felt, flow-through electrode. J. Electrochem. Soc. 1982, 129, 1993. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.; Yang, Y.-S.; Wen, Y.-H.; Wang, X.-D.; Cao, G.-P. Study of zinc electrodes for single flow zinc/nickel battery application. J. Power Sources 2008, 179, 381–387. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Cheng, J.; Ning, S.-Q.; Yang, Y.-S. Preliminary study on zinc–air battery using zinc regeneration electrolysis with propanol oxidation as a counter electrode reaction. J. Power Sources 2009, 188, 301–307. [Google Scholar] [CrossRef]
- Gu, S.; Gong, K.; Yan, E.Z.; Yan, Y. A multiple ion-exchange membrane design for redox flow batteries. Energy Environ. Sci. 2014, 7, 2986–2998. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, L.; Yang, Y.-S.; Wen, Y.-H.; Cao, G.-P.; Wang, X.-D. Preliminary study of single flow zinc–nickel battery. Electrochem. Commun. 2007, 9, 2639–2642. [Google Scholar] [CrossRef]
- Gong, K.; Fang, Q.; Gu, S.; Li, S.F.Y.; Yan, Y. Nonaqueous redox-flow batteries: Organic solvents, supporting electrolytes, and redox pairs. Energy Environ. Sci. 2015, 8, 3515–3530. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Ryu, J.; Cho, J. Nanostructured electrocatalysts for all-vanadium redox flow batteries. Chem.-Asian J. 2015, 10, 2096–2110. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Brandon, N.; Hajimolana, S.; Tariq, F.; Yufit, V.; Hashim, M.; Hussain, M.; Low, C.; Aravind, P. Application of carbon materials in redox flow batteries. J. Power Sources 2014, 253, 150–166. [Google Scholar] [CrossRef]
- Ngamsai, K.; Arpornwichanop, A. Study on mechanism and kinetic of air oxidation of V (II) in electrolyte reservoir of a vanadium redox flow battery. Energy Procedia 2014, 61, 1642–1645. [Google Scholar] [CrossRef] [Green Version]
- Leung, P.; Li, X.; De León, C.P.; Berlouis, L.; Low, C.J.; Walsh, F.C. Progress in redox flow batteries, remaining challenges and their applications in energy storage. Rsc Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M. Novel vanadium chloride/polyhalide redox flow battery. J. Power Sources 2003, 124, 299–302. [Google Scholar] [CrossRef]
- Kazacos, M.; Cheng, M.; Skyllas-Kazacos, M. Vanadium redox cell electrolyte optimization studies. J. Appl. Electrochem. 1990, 20, 463–467. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Nie, Z.; Chen, B.; Vijayakumar, M.; Kim, S.; Wang, W.; Schwenzer, B.; Liu, J.; Yang, Z. Effects of additives on the stability of electrolytes for all-vanadium redox flow batteries. J. Appl. Electrochem. 2011, 41, 1215–1221. [Google Scholar] [CrossRef]
- Mohamed, M.; Leung, P.; Sulaiman, M. Performance characterization of a vanadium redox flow battery at different operating parameters under a standardized test-bed system. Appl. Energy 2015, 137, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Islam, R.; Nolen, C.; Jeong, K. Effects of Sulfuric Acid Concentration on Volume Transfer Across Ion-Exchange Membrane in a Single-Cell Vanadium Redox Flow Battery. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Tampa, FL, USA, 3–9 November 2019; American Society of Mechanical Engineers: New York, NY, USA, 2017; Volume 58417, p. V006T008A025. [Google Scholar]
- Peng, S.; Wang, N.-F.; Wu, X.-J.; Liu, S.-Q.; Fang, D.; Liu, Y.-N.; Huang, K.-L. Vanadium species in CH3SO3H and H2SO4 mixed acid as the supporting electrolyte for vanadium redox flow battery. Int. J. Electrochem. Sci. 2012, 7, 643–649. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, C.; Ren, H.; Zhang, Y.; Ma, C. Electrochemical performance of VO2+/VO2+ redox couple in the H2SO4-CH3SO3H solutions. Int. J. Electrochem. Sci 2016, 11, 1987–1996. [Google Scholar] [CrossRef]
- Roznyatovskaya, N.; Noack, J.; Mild, H.; Fühl, M.; Fischer, P.; Pinkwart, K.; Tübke, J.; Skyllas-Kazacos, M. Vanadium electrolyte for all-vanadium redox-flow batteries: The effect of the counter ion. Batteries 2019, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Rahman, F.; Skyllas-Kazacos, M. Vanadium redox battery: Positive half-cell electrolyte studies. J. Power Sources 2009, 189, 1212–1219. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Kazacos, G.; Poon, G.; Verseema, H. Recent advances with UNSW vanadium-based redox flow batteries. Int. J. Energy Res. 2010, 34, 182–189. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.J.; Lee, H.; Kim, H. Development of a redox flow battery with multiple redox couples at both positive and negative electrolytes for high energy density. J. Electrochem. Soc. 2018, 165, A3215. [Google Scholar] [CrossRef]
- Menictas, C.; Skyllas-Kazacos, M. Performance of vanadium-oxygen redox fuel cell. J. Appl. Electrochem. 2011, 41, 1223. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Lindfield Roberts, E.P.; Saleem, M.J. Charge–discharge performance of a novel undivided redox flow battery for renewable energy storage. Int. J. Green Energy 2010, 7, 445–460. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Dryfe, R.; Roberts, E. Evaluation of electrolytes for redox flow battery applications. Electrochim. Acta 2007, 52, 2189–2195. [Google Scholar] [CrossRef]
- Matsuda, Y.; Tanaka, K.; Okada, M.; Takasu, Y.; Morita, M.; Matsumura-Inoue, T. A rechargeable redox battery utilizing ruthenium complexes with non-aqueous organic electrolyte. J. Appl. Electrochem. 1988, 18, 909–914. [Google Scholar] [CrossRef]
- Clark, R. Flow batteries and energy storage—A new market for ceramics. Am. Ceram. Soc. Bull. 2022, 101, 22–27. [Google Scholar]
- Zhang, L.; Feng, R.; Wang, W.; Yu, G. Emerging chemistries and molecular designs for flow batteries. Nat. Rev. Chem. 2022, 6, 524–543. [Google Scholar] [CrossRef] [PubMed]
- Alotto, P.; Guarnieri, M.; Moro, F.; Stella, A. Redox Flow Batteries for large scale energy storage. In Proceedings of the 2012 IEEE International Energy Conference and Exhibition (ENERGYCON), Florence, Italy, 9–12 September 2012; pp. 293–298. [Google Scholar] [CrossRef]
- Rychcik, M.; Skyllas-Kazacos, M. Characteristics of a new all-vanadium redox flow battery. J. Power Sources 1988, 22, 59–67. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review of the development of first-generation redox flow batteries: Iron-chromium system. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef] [PubMed]
- Robb, B.H.; Farrell, J.M.; Marshak, M.P. Chelated Chromium Electrolyte Enabling High-Voltage Aqueous Flow Batteries. Joule 2019, 3, 2503–2512. [Google Scholar] [CrossRef]
- Atawi, I.E.; Al-Shetwi, A.Q.; Magableh, A.M.; Albalawi, O.H. Recent Advances in Hybrid Energy Storage System Integrated Renewable Power Generation: Configuration, Control, Applications, and Future Directions. Batteries 2022, 9, 29. [Google Scholar] [CrossRef]
- Han, D.; Shanmugam, S. Active material crossover suppression with bi-ionic transportability by an amphoteric membrane for Zinc–Bromine redox flow battery. J. Power Sources 2022, 540, 231637. [Google Scholar] [CrossRef]
- Li, X.; Li, N.; Huang, Z.; Chen, Z.; Zhao, Y.; Liang, G.; Yang, Q.; Li, M.; Huang, Q.; Dong, B. Confining aqueous Zn–Br halide redox chemistry by Ti3C2TX MXene. ACS Nano 2021, 15, 1718–1726. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, C.; Zhang, H.; Li, X. Anode for zinc-based batteries: Challenges, strategies, and prospects. ACS Energy Lett. 2021, 6, 2765–2785. [Google Scholar] [CrossRef]
- Yu, F.; Pang, L.; Wang, X.; Waclawik, E.R.; Wang, F.; Ostrikov, K.K.; Wang, H.J. Aqueous alkaline–acid hybrid electrolyte for zinc-bromine battery with 3V voltage window. Energy Storage Mater. 2019, 19, 56–61. [Google Scholar] [CrossRef]
- Niu, G. Electric-Hydraulic Hybrid Energy Storage System for Electrified Transportation; Illinois Institute of Technology: Chicago, IL, USA, 2017. [Google Scholar]
- Kear, G.; Shah, A.A.; Walsh, F. Development of the all-vanadium redox flow battery for energy storage: A review of technological, financial and policy aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef] [Green Version]
- Rajarathnam, G.P.; Vassallo, A.M.; Rajarathnam, G.P.; Vassallo, A.; Advancement, P. Strategies for Studying and Improving the Zn/Br RFB. In The Zinc/Bromine Flow Battery: Materials Challenges and Practical Solutions for Technology Advancement; Springer: Berlin/Heidelberg, Germany, 2016; pp. 81–97. [Google Scholar]
- Ruan, W.; Mao, J.; Yang, S.; Shi, C.; Jia, G.; Chen, Q. Designing Cr complexes for a neutral Fe–Cr redox flow battery. Chem. Commun. 2020, 56, 3171–3174. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.-y.; Yang, J.H.; Kim, H.-T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Janoschka, T.; Hager, M.D.; Schubert, U.S. Powering up the future: Radical polymers for battery applications. Adv. Mater. 2012, 24, 6397–6409. [Google Scholar] [CrossRef]
- Nishide, H.; Koshika, K.; Oyaizu, K. Environmentally benign batteries based on organic radical polymers. Pure Appl. Chem. 2009, 81, 1961–1970. [Google Scholar] [CrossRef]
- Oyaizu, K.; Kawamoto, T.; Suga, T.; Nishide, H. Synthesis and charge transport properties of redox-active nitroxide polyethers with large site density. Macromolecules 2010, 43, 10382–10389. [Google Scholar] [CrossRef]
- Nakahara, K.; Iriyama, J.; Iwasa, S.; Suguro, M.; Satoh, M.; Cairns, E.J. High-rate capable organic radical cathodes for lithium rechargeable batteries. J. Power Sources 2007, 165, 870–873. [Google Scholar] [CrossRef]
- Nesvadba, P.; Bugnon, L.; Maire, P.; Novák, P. Synthesis of a novel spirobisnitroxide polymer and its evaluation in an organic radical battery. Chem. Mater. 2010, 22, 783–788. [Google Scholar] [CrossRef]
- Schon, T.B.; McAllister, B.T.; Li, P.-F.; Seferos, D.S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Assary, R.S.; Qu, X.; Jain, A.; Ong, S.P.; Rajput, N.N.; Persson, K.; Curtiss, L.A. Accelerating electrolyte discovery for energy storage with high-throughput screening. J. Phys. Chem. Lett. 2015, 6, 283–291. [Google Scholar] [CrossRef]
- Pineda Flores, S.D.; Martin-Noble, G.C.; Phillips, R.L.; Schrier, J. Bio-Inspired electroactive organic molecules for aqueous redox flow batteries. 1. Thiophenoquinones. J. Phys. Chem. C 2015, 119, 21800–21809. [Google Scholar] [CrossRef]
- Moon, Y.; Han, Y.-K. Computational screening of organic molecules as redox active species in redox flow batteries. Curr. Appl. Phys. 2016, 16, 939–943. [Google Scholar] [CrossRef]
- Zhong, F.; Yang, M.; Ding, M.; Jia, C. Organic electroactive molecule-based electrolytes for redox flow batteries: Status and challenges of molecular design. Front. Chem. 2020, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Ventosa, E. Semi-solid flow battery and redox-mediated flow battery: Two strategies to implement the use of solid electroactive materials in high-energy redox-flow batteries. Curr. Opin. Chem. Eng. 2022, 37, 100834. [Google Scholar] [CrossRef]
- Duduta, M.; Ho, B.; Wood, V.C.; Limthongkul, P.; Brunini, V.E.; Carter, W.C.; Chiang, Y.M. Semi-solid lithium rechargeable flow battery. Adv. Energy Mater. 2011, 1, 511–516. [Google Scholar] [CrossRef]
- Ventosa, E.; Zampardi, G.; Flox, C.; La Mantia, F.; Schuhmann, W.; Morante, J. Solid electrolyte interphase in semi-solid flow batteries: A wolf in sheep’s clothing. Chem. Commun. 2015, 51, 14973–14976. [Google Scholar] [CrossRef] [Green Version]
- Mourshed, M.; Niya, S.M.R.; Ojha, R.; Rosengarten, G.; Andrews, J.; Shabani, B. Carbon-based slurry electrodes for energy storage and power supply systems. Energy Storage Mater. 2021, 40, 461–489. [Google Scholar] [CrossRef]
- Debruler, C.; Wu, W.; Cox, K.; Vanness, B.; Liu, T. Integrated saltwater desalination and energy storage through a pH neutral aqueous organic redox flow battery. Adv. Funct. Mater. 2020, 30, 2000385. [Google Scholar] [CrossRef]
- Madec, L.; Youssry, M.; Cerbelaud, M.; Soudan, P.; Guyomard, D.; Lestriez, B. Surfactant for enhanced rheological, electrical, and electrochemical performance of suspensions for semisolid redox flow batteries and supercapacitors. ChemPlusChem 2015, 80, 396. [Google Scholar] [CrossRef]
- Wei, T.S.; Fan, F.Y.; Helal, A.; Smith, K.C.; McKinley, G.H.; Chiang, Y.M.; Lewis, J.A. Biphasic Electrode Suspensions for Li-Ion Semi-solid Flow Cells with High Energy Density, Fast Charge Transport, and Low-Dissipation Flow. Adv. Energy Mater. 2015, 5, 1500535. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.; Pan, F.; Zhu, Y.G.; Huang, Q.; Lu, L.; Wang, Q. High–energy density nonaqueous all redox flow lithium battery enabled with a polymeric membrane. Sci. Adv. 2015, 1, e1500886. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Chen, Y.; Zhang, Q.; Xi, S.; Yu, J.; Du, Y.; Hu, Y.; Wang, Q. Na3V2 (PO4)3 as the Sole Solid Energy Storage Material for Redox Flow Sodium–Ion Battery. Adv. Energy Mater. 2019, 9, 1901188. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Du, Y.; Jia, C.; Zhou, M.; Fan, L.; Wang, X.; Wang, Q. Unleashing the power and energy of LiFePO4-based redox flow lithium battery with a bifunctional redox mediator. J. Am. Chem. Soc. 2017, 139, 6286–6289. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Huang, S.; Samarakoon, W.; Xi, S.; Ji, Y.; Zhang, H.; Zhang, F.; Du, Y.; Feng, Z. Redox targeting-based vanadium redox-flow battery. ACS Energy Lett. 2019, 4, 3028–3035. [Google Scholar] [CrossRef]
- Yu, J.; Fan, L.; Yan, R.; Zhou, M.; Wang, Q. Redox targeting-based aqueous redox flow lithium battery. ACS Energy Lett. 2018, 3, 2314–2320. [Google Scholar] [CrossRef]
- Zanzola, E.; Dennison, C.R.; Battistel, A.; Peljo, P.; Vrubel, H.; Amstutz, V.; Girault, H.H. Redox solid energy boosters for flow batteries: Polyaniline as a case study. Electrochim. Acta 2017, 235, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Zanzola, E.; Gentil, S.; Gschwend, G.; Reynard, D.; Smirnov, E.; Dennison, C.; Girault, H.H.; Peljo, P. Solid electrochemical energy storage for aqueous redox flow batteries: The case of copper hexacyanoferrate. Electrochim. Acta 2019, 321, 134704. [Google Scholar] [CrossRef]
- Páez, T.; Martínez-Cuezva, A.; Palma, J.S.; Ventosa, E. Mediated alkaline flow batteries: From fundamentals to application. ACS Appl. Energy Mater. 2019, 2, 8328–8336. [Google Scholar] [CrossRef]
- Liu, P.; Cao, Y.l.; Li, G.R.; Gao, X.P.; Ai, X.P.; Yang, H.X. A solar rechargeable flow battery based on photoregeneration of two soluble redox couples. ChemSusChem 2013, 6, 802–806. [Google Scholar] [CrossRef]
- Yan, N.; Li, G.; Gao, X. Solar rechargeable redox flow battery based on Li2WO4/LiI couples in dual-phase electrolytes. J. Mater. Chem. A 2013, 1, 7012–7015. [Google Scholar] [CrossRef]
- Yan, N.; Li, G.; Gao, X. Electroactive organic compounds as anode-active materials for solar rechargeable redox flow battery in dual-phase electrolytes. J. Electrochem. Soc. 2014, 161, A736. [Google Scholar] [CrossRef]
- Yu, M.; McCulloch, W.D.; Beauchamp, D.R.; Huang, Z.; Ren, X.; Wu, Y. Aqueous lithium–iodine solar flow battery for the simultaneous conversion and storage of solar energy. J. Am. Chem. Soc. 2015, 137, 8332–8335. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Seipp, T.; Burfeind, J.; Sousa, C.; Bentien, A.; Araújo, J.P.; Mendes, A. Unbiased solar energy storage: Photoelectrochemical redox flow battery. Nano Energy 2016, 22, 396–405. [Google Scholar] [CrossRef]
- Liao, S.; Zong, X.; Seger, B.; Pedersen, T.; Yao, T.; Ding, C.; Shi, J.; Chen, J.; Li, C. Integrating a dual-silicon photoelectrochemical cell into a redox flow battery for unassisted photocharging. Nat. Commun. 2016, 7, 11474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedege, K.; Azevedo, J.; Khataee, A.; Bentien, A.; Mendes, A. Direct solar charging of an organic–inorganic, stable, and aqueous alkaline redox flow battery with a hematite photoanode. Angew. Chem. Int. Ed. 2016, 55, 7142–7147. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, W.D.; Yu, M.; Wu, Y. pH-tuning a solar redox flow battery for integrated energy conversion and storage. ACS Energy Lett. 2016, 1, 578–582. [Google Scholar] [CrossRef]
- Li, W.; Fu, H.C.; Li, L.; Cabán-Acevedo, M.; He, J.H.; Jin, S. Integrated photoelectrochemical solar energy conversion and organic redox flow battery devices. Angew. Chem. 2016, 128, 13298–13302. [Google Scholar] [CrossRef]
- McKone, J.R.; DiSalvo, F.J.; Abruña, H.D. Solar energy conversion, storage, and release using an integrated solar-driven redox flow battery. J. Mater. Chem. A 2017, 5, 5362–5372. [Google Scholar] [CrossRef]
- Cheng, Q.; Fan, W.; He, Y.; Ma, P.; Vanka, S.; Fan, S.; Mi, Z.; Wang, D. Photorechargeable high voltage redox battery enabled by Ta3N5 and GaN/Si dual-photoelectrode. Adv. Mater. 2017, 29, 1700312. [Google Scholar] [CrossRef]
- Wedege, K.; Bae, D.; Dražević, E.; Mendes, A.; Vesborg, P.C.; Bentien, A. Unbiased, complete solar charging of a neutral flow battery by a single Si photocathode. RSC Adv. 2018, 8, 6331–6340. [Google Scholar] [CrossRef]
- Liao, S.; Shi, J.; Ding, C.; Liu, M.; Xiong, F.; Wang, N.; Chen, J.; Li, C. Photoelectrochemical regeneration of all vanadium redox species for construction of a solar rechargeable flow cell. J. Energy Chem. 2018, 27, 278–282. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Fu, H.-C.; Zhao, Y.; He, J.-H.; Jin, S. 14.1% efficient monolithically integrated solar flow battery. Chem 2018, 4, 2644–2657. [Google Scholar] [CrossRef] [Green Version]
- Urbain, F.; Murcia-López, S.; Nembhard, N.; Vázquez-Galván, J.; Flox, C.; Smirnov, V.; Welter, K.; Andreu, T.; Finger, F.; Morante, J.R. Solar vanadium redox-flow battery powered by thin-film silicon photovoltaics for efficient photoelectrochemical energy storage. J. Phys. D Appl. Phys. 2018, 52, 044001. [Google Scholar] [CrossRef] [Green Version]
- Murcia-López, S.; Chakraborty, M.; Carretero, N.M.; Flox, C.; Morante, J.R.; Andreu, T. Adaptation of Cu (In, Ga) Se2 photovoltaics for full unbiased photocharge of integrated solar vanadium redox flow batteries. Sustain. Energy Fuels 2020, 4, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Pan, M.S.; Su, L.; Tsai, P.-C.; Badel, A.F.; Valle, J.M.; Eiler, S.L.; Xiang, K.; Brushett, F.R.; Chiang, Y.-M. Air-breathing aqueous sulfur flow battery for ultralow-cost long-duration electrical storage. Joule 2017, 1, 306–327. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, H.S.; Bandal, H.A.; Ramakrishna, S.; Kim, H. Critical review, recent updates on zeolitic imidazolate framework-67 (ZIF-67) and its derivatives for electrochemical water splitting. Adv. Mater. 2022, 34, 2107072. [Google Scholar] [CrossRef]
- Pan, M.S. Aqueous Polysulfide Electrodes for Low-Cost Grid-Scale Energy Storage. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2022. [Google Scholar]
- Wang, K.; Wu, Y.; Cao, X.; Gu, L.; Hu, J. A Zn–CO2 flow battery generating electricity and methane. Adv. Funct. Mater. 2020, 30, 1908965. [Google Scholar] [CrossRef]
- Chen, Y.; Mei, Y.; Li, M.; Dang, C.; Huang, L.; Wu, W.; Wu, Y.; Yu, X.; Wang, K.; Gu, L.; et al. Highly selective CO2 conversion to methane or syngas tuned by CNTs@non-noble–metal cathodes in Zn–CO2 flow batteries. Green Chem. 2021, 23, 8138–8146. [Google Scholar] [CrossRef]
- Hosseini-Benhangi, P.; Gyenge, C.C.; Gyenge, E.L. The carbon dioxide redox flow battery: Bifunctional CO2 reduction/formate oxidation electrocatalysis on binary and ternary catalysts. J. Power Sources 2021, 495, 229752. [Google Scholar] [CrossRef]
- Liu, F.; Ma, Z.; Liu, Q.; Wang, Z.; He, C. An integrated solar redox flow battery using a single Si photoanode and near-neutral electrolytes. J. Power Sources 2022, 549, 231987. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y. Recent Development of CO2 Electrochemistry from Li–CO2 Batteries to Zn–CO2 Batteries. Acc. Chem. Res. 2019, 52, 1721–1729. [Google Scholar] [CrossRef]
- Bae, C.-H.; Roberts, E.; Dryfe, R. Chromium redox couples for application to redox flow batteries. Electrochim. Acta 2002, 48, 279–287. [Google Scholar] [CrossRef]
- Doria, J.; De Andres, M.; Armenta, C. All-chromium redox couple for battery application. In Proceedings of the INTERSOL 85 Proceedings of the Ninth Biennial Congress of the International Solar Energy Society, Montreal, Canada, 23–29 June 1985; Volume 3. [Google Scholar]
- Codina, G.; Perez, J.; Lopez-Atalaya, M.; Vasquez, J.; Aldaz, A. Development of a 0.1 kW power accumulation pilot plant based on an Fe/Cr redox flow battery Part I. Considerations on flow-distribution design. J. Power Sources 1994, 48, 293–302. [Google Scholar] [CrossRef]
- Garces, P.; Climent, M.; Aldaz, A. Systems for Storage of Electrical Energy. 1. 1st Results of a Redox Battery. An. Quim. Ser. A-Quim. Fis. Quim. Tec. 1987, 83, 9–11. [Google Scholar]
- Qiao, L.; Fang, M.; Liu, S.; Zhang, H.; Ma, X. New-generation iron–titanium flow batteries with low cost and ultrahigh stability for stationary energy storage. Chem. Eng. J. 2022, 434, 134588. [Google Scholar] [CrossRef]
- Fang, B.; Iwasa, S.; Wei, Y.; Arai, T.; Kumagai, M. A study of the Ce(III)/Ce(IV) redox couple for redox flow battery application. Electrochim. Acta 2002, 47, 3971–3976. [Google Scholar] [CrossRef]
- Greco, K.V.; Forner-Cuenca, A.; Mularczyk, A.; Eller, J.; Brushett, F.R. Elucidating the Nuanced Effects of Thermal Pretreatment on Carbon Paper Electrodes for Vanadium Redox Flow Batteries. ACS Appl. Mater. Interfaces 2018, 10, 44430–44442. [Google Scholar] [CrossRef]
- Weber, A.Z.; Borup, R.L.; Darling, R.M.; Das, P.K.; Dursch, T.J.; Gu, W.; Harvey, D.; Kusoglu, A.; Litster, S.; Mench, M.M. A critical review of modeling transport phenomena in polymer-electrolyte fuel cells. J. Electrochem. Soc. 2014, 161, F1254. [Google Scholar] [CrossRef] [Green Version]
- Ashraf Gandomi, Y.; Aaron, D.S.; Mench, M.M. Influence of membrane equivalent weight and reinforcement on ionic species crossover in all-vanadium redox flow batteries. Membranes 2017, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Blanc, C.; Rufer, A. Optimization of the operating point of a vanadium redox flow battery. In Proceedings of the Energy Conversion Congress & Exposition, San Jose, CA, USA, 20–24 September 2009; IEEE: New York, NY, USA, 2009. [Google Scholar]
- Gandomi, Y.A.; Edmundson, M.; Busby, F.; Mench, M. Water management in polymer electrolyte fuel cells through asymmetric thermal and mass transport engineering of the micro-porous layers. J. Electrochem. Soc. 2016, 163, F933. [Google Scholar] [CrossRef] [Green Version]
- Gandomi, Y.A.; Mench, M. Assessing the limits of water management using asymmetric micro-porous layer configurations. ECS Trans. 2013, 58, 1375. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, T.; An, L.; Zhou, X.; Wei, L. A comparative study of all-vanadium and iron-chromium redox flow batteries for large-scale energy storage. J. Power Sources 2015, 300, 438–443. [Google Scholar] [CrossRef]
- Houser, J.; Clement, J.; Pezeshki, A.; Mench, M.M. Influence of architecture and material properties on vanadium redox flow battery performance. J. Power Sources 2016, 302, 369–377. [Google Scholar] [CrossRef]
- Zhang, M.; Moore, M.; Watson, J.; Zawodzinski, T.A.; Counce, R.M. Capital cost sensitivity analysis of an all-vanadium redox-flow battery. J. Electrochem. Soc. 2012, 159, A1183. [Google Scholar] [CrossRef]
- Eckroad, S. Renewables, EPRI-1014836; Electric Power Research Institute: Palo Alto, CA, USA, 2007. [Google Scholar]
- Aaron, D.; Yeom, S.; Kihm, K.D.; Gandomi, Y.A.; Ertugrul, T.; Mench, M.M. Kinetic enhancement via passive deposition of carbon-based nanomaterials in vanadium redox flow batteries. J. Power Sources 2017, 366, 241–248. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M. Vanadium/Polyhalide Redox Flow Battery. U.S. Patent US 7,320,844 B2, 22 January 2008. [Google Scholar]
- Knehr, K.; Agar, E.; Dennison, C.; Kalidindi, A.; Kumbur, E. A transient vanadium flow battery model incorporating vanadium crossover and water transport through the membrane. J. Electrochem. Soc. 2012, 159, A1446. [Google Scholar] [CrossRef]
- Knehr, K.; Kumbur, E. Open circuit voltage of vanadium redox flow batteries: Discrepancy between models and experiments. Electrochem. Commun. 2011, 13, 342–345. [Google Scholar] [CrossRef]
- Chalamala, B.R.; Soundappan, T.; Fisher, G.R.; Anstey, M.R.; Viswanathan, V.V.; Perry, M.L. Redox flow batteries: An engineering perspective. Proc. IEEE 2014, 102, 976–999. [Google Scholar] [CrossRef]
- Ayala-Charca, G.; Forouhi, S.; Zoidl, G.; Magierowski, S.; Ghafar-Zadeh, E. Resonance-Like Impedance Measurement Technique for Life Science Applications. IEEE Trans. Instrum. Meas. 2022, 71, 1–11. [Google Scholar] [CrossRef]
- Friedl, J.; Bauer, C.M.; Rinaldi, A.; Stimming, U. Electron transfer kinetics of the VO2+/VO2+—Reaction on multi-walled carbon nanotubes. Carbon 2013, 63, 228–239. [Google Scholar] [CrossRef]
- Kim, T.; Choi, W.; Shin, H.-C.; Choi, J.-Y.; Kim, J.M.; Park, M.-S.; Yoon, W.-S. Applications of voltammetry in lithium ion battery research. J. Electrochem. Sci. Technol. 2020, 11, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Natu, V.; Benchakar, M.; Canaff, C.; Habrioux, A.; Célérier, S.; Barsoum, M.W. A critical analysis of the X-ray photoelectron spectra of Ti3C2Tz MXenes. Matter 2021, 4, 1224–1251. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Breitkopf, C. Determination of diffusion coefficients using impedance spectroscopy data. J. Electrochem. Soc. 2018, 165, E826–E831. [Google Scholar] [CrossRef]
- Giaccherini, A.; Al Khatib, M.; Cinotti, S.; Piciollo, E.; Berretti, E.; Giusti, P.; Innocenti, M.; Montegrossi, G.; Lavacchi, A. Analysis of mass transport in ionic liquids: A rotating disk electrode approach. Sci. Rep. 2020, 10, 13433. [Google Scholar] [CrossRef]
- Liao, H.; Watson, W.; Dizon, A.; Tribollet, B.; Vivier, V.; Orazem, M.E. Physical properties obtained from measurement model analysis of impedance measurements. Electrochim. Acta 2020, 354, 136747. [Google Scholar] [CrossRef]
- Sun, C.-N.; Delnick, F.M.; Aaron, D.; Papandrew, A.; Mench, M.M.; Zawodzinski, T., Jr. Probing electrode losses in all-vanadium redox flow batteries with impedance spectroscopy. ECS Electrochem. Lett. 2013, 2, A43. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Kowalski, J.A.; Carroll, K.J.; Brushett, F.R. Recent developments and trends in redox flow batteries. In Rechargeable Batteries; Springer: Berlin/Heidelberg, Germany, 2015; pp. 673–712. [Google Scholar]
- Ulaganathan, M.; Aravindan, V.; Yan, Q.; Madhavi, S.; Skyllas-Kazacos, M.; Lim, T.M. Recent advancements in all-vanadium redox flow batteries. Adv. Mater. Interfaces 2016, 3, 1500309. [Google Scholar] [CrossRef]
- Cheng, M. Electrolyte Optimization and Studies for the Vanadium Redox Battery. Master’s Thesis, The University of New South Wales, Sydney, Australia, 1991. [Google Scholar]
- Aaron, D.; Liu, Q.; Tang, Z.; Grim, G.; Papandrew, A.; Turhan, A.; Zawodzinski, T.; Mench, M. Dramatic performance gains in vanadium redox flow batteries through modified cell architecture. J. Power Sources 2012, 206, 450–453. [Google Scholar] [CrossRef]
- Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G.; Hu, J.; Graff, G. A stable vanadium redox-flow battery with high energy density for large-scale energy storage. Adv. Energy Mater. 2011, 1, 394–400. [Google Scholar] [CrossRef]
- Livshits, V.; Ulus, A.; Peled, E. High-power H2/Br2 fuel cell. Electrochem. Commun. 2006, 8, 1358–1362. [Google Scholar] [CrossRef]
- Cho, K.T.; Ridgway, P.; Weber, A.Z.; Haussener, S.; Battaglia, V.; Srinivasan, V. High performance hydrogen/bromine redox flow battery for grid-scale energy storage. J. Electrochem. Soc. 2012, 159, A1806. [Google Scholar] [CrossRef]
- Cho, K.T.; Albertus, P.; Battaglia, V.; Kojic, A.; Srinivasan, V.; Weber, A.Z. Optimization and analysis of high-power hydrogen/bromine-flow batteries for grid-scale energy storage. Energy Technol. 2013, 1, 596–608. [Google Scholar] [CrossRef]
- Darling, R.M.; Perry, M.L. The influence of electrode and channel configurations on flow battery performance. J. Electrochem. Soc. 2014, 161, A1381. [Google Scholar] [CrossRef]
- Cho, K.T.; Tucker, M.C.; Ding, M.; Ridgway, P.; Battaglia, V.S.; Srinivasan, V.; Weber, A.Z. Cyclic Performance Analysis of Hydrogen/Bromine Flow Batteries for Grid-Scale Energy Storage. ChemPlusChem 2015, 80, 402–411. [Google Scholar] [CrossRef]
- Lin, G.; Chong, P.Y.; Yarlagadda, V.; Nguyen, T.; Wycisk, R.; Pintauro, P.; Bates, M.; Mukerjee, S.; Tucker, M.; Weber, A.Z. Advanced hydrogen-bromine flow batteries with improved efficiency, durability and cost. J. Electrochem. Soc. 2015, 163, A5049. [Google Scholar] [CrossRef] [Green Version]
- Talaie, E.; Bonnick, P.; Sun, X.; Pang, Q.; Liang, X.; Nazar, L.F. Methods and Protocols for Electrochemical Energy Storage Materials Research. Chem. Mater. 2016, 29, 90–105. [Google Scholar] [CrossRef]
- Peltier, C.R.; Rhodes, Z.; Macbeth, A.J.; Milam, A.; Carroll, E.; Coates, G.W.; Minteer, S.D. Suppressing Crossover in Nonaqueous Redox Flow Batteries with Polyethylene-Based Anion-Exchange Membranes. ACS Energy Lett. 2022, 7, 4118–4128. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef]
- Goulet, M.-A.; Kjeang, E. Co-laminar flow cells for electrochemical energy conversion. J. Power Sources 2014, 260, 186–196. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2017, 95, 197–206. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2017, 122, 194–206. [Google Scholar] [CrossRef]
- Liu, X.; Li, K.; Li, X. The Electrochemical Performance and Applications of Several Popular Lithium-ion Batteries for Electric Vehicles—Preparation and Characterization.pdf A Review. In Advances in Green Energy Systems and Smart Grid; Communications in Computer and Information Science; Springer: Singapore, 2018; pp. 201–213. [Google Scholar]
- Major, G.H.; Fairley, N.; Sherwood, P.M.A.; Linford, M.R.; Terry, J.; Fernandez, V.; Artyushkova, K. Practical guide for curve fitting in X-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 061203. [Google Scholar] [CrossRef]
- Dattaguru, A. Summary of Some Selected Characterization Methods of Geopolymers. In Geopolymers and Other Geosynthetics; Mazen, A., Han-Yong, J., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 3. [Google Scholar]
- Makansi, J.; Abboud, J. Energy Storage: The Missing Link in the U.S. Electricity Value Chain-An ESC White Paper. Energy Storage Council May; Research Gate: Berlin, Germany, 2002; pp. 1–23. [Google Scholar]
- Menictas, C.; Hong, D.; Yah, Z.; Wilson, J.; Kazacos, M.; Skyllas-Kazacos, M. Status of the vanadium redox battery development program. In National Conference Publication-Institution of Engineers Australia NCP; Institution of Engineers: Barton, Australia, 1994; pp. 299–304. [Google Scholar]
- Weber, S.; Peters, J.F.; Baumann, M.; Weil, M. Life cycle assessment of a vanadium redox flow battery. Environ. Sci. Technol. 2018, 52, 10864–10873. [Google Scholar] [CrossRef]
- Pritil, G.; Maria, C.; Power, D. Vanadium Redox Flow Batteries Identifiying Market Opportunities and Enablers; Guidehouse INSIGHTS: London, UK, 2022. [Google Scholar]
- Arenas, L.; De León, C.P.; Walsh, F. Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef] [Green Version]
- Pickett, D.J. Electrochemical Reactor Design; Elsevier Science Limited: Amsterdam, The Netherlands, 1979; Volume 9. [Google Scholar]
- Pletcher, D.; Walsh, F.C. Industrial Electrochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Walsh, F.C. A First Course in Electrochemical Engineering; Electrochemical Consultancy: Romsey, UK, 1993. [Google Scholar]
- Maeda, S.; Sugawara, J.; Hayami, H. Bipolar Plate for Redox Flow Battery. U.S. Patent Application No. 13/641,651, 14 February 2013. [Google Scholar]
- Horne, C.R.; Kinoshita, K.; Hickey, D.B. Redox Flow Battery System for Distributed Energy Storage. U.S. Patent Application No. 12/498,103, 26 October 2010. [Google Scholar]
- Lex, P.; Coad, N. Battery Flow Frame Material Formulation. U.S. Patent Application No. 14/182,487, 12 June 2014. [Google Scholar]
- Newman, J.; Thomas-Alyea, K. Electrochemical Systems, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Chakrabarti, B.K.; Kalamaras, E.; Singh, A.K.; Bertei, A.; Rubio-Garcia, J.; Yufit, V. Modelling of redox flow battery electrode processes at a range of length scales: A review. Sustain. Energy Fuels 2020, 4, 5433–5468. [Google Scholar] [CrossRef]
- Kuhn, A.; Booth, J. Electrical leakage currents in bipolar cell stacks. J. Appl. Electrochem. 1980, 10, 233–237. [Google Scholar] [CrossRef]
- Roušar, I.; Cezner, V. Experimental determination and calculation of parasitic currents in bipolar electrolyzers with application to chlorate electrolyzer. J. Electrochem. Soc. 1974, 121, 648. [Google Scholar] [CrossRef]
- Xing, F.; Zhang, H.; Ma, X.J. Shunt current loss of the vanadium redox flow battery. J. Power Sources 2011, 196, 10753–10757. [Google Scholar] [CrossRef]
- Tang, A.; McCann, J.; Bao, J.; Skyllas-Kazacos, M.J. Investigation of the effect of shunt current on battery efficiency and stack temperature in vanadium redox flow battery. J. Power Sources 2013, 242, 349–356. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Ho, S.-Y.; Chou, H.-W.; Wei, H.-J. Modeling the effect of shunt current on the charge transfer efficiency of an all-vanadium redox flow battery. J. Power Sources 2018, 390, 168–175. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.; Xu, Q.; Zhou, X.; Zhang, Z. In-situ investigation of hydrogen evolution behavior in vanadium redox flow batteries. Appl. Energy 2017, 190, 1112–1118. [Google Scholar] [CrossRef]
- Shah, A.; Al-Fetlawi, H.; Walsh, F. Dynamic modelling of hydrogen evolution effects in the all-vanadium redox flow battery. Electrochim. Acta 2010, 55, 1125–1139. [Google Scholar] [CrossRef] [Green Version]
- Al-Fetlawi, H.; Shah, A.; Walsh, F. Modelling the effects of oxygen evolution in the all-vanadium redox flow battery. Electrochim. Acta 2010, 55, 3192–3205. [Google Scholar] [CrossRef] [Green Version]
- Chang, O.K.; Pham, A.Q. Rebalancing Electrolytes in Redox Flow Battery Systems. U.S. Patent No. 8,916,281, 23 December 2014. [Google Scholar]
- Cao, L.; Skyllas-Kazacos, M.; Menictas, C.; Noack, J. A review of electrolyte additives and impurities in vanadium redox flow batteries. J. Energy Chem. 2018, 27, 1269–1291. [Google Scholar] [CrossRef]
- Wan, C.T.-C.; Rodby, K.E.; Perry, M.L.; Chiang, Y.-M.; Brushett, F. Hydrogen evolution mitigation in iron-chromium redox flow batteries via electrochemical purification of the electrolyte. J. Power Sources 2023, 554, 232248. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, T.; Zhou, X.; Zou, J.; Ren, Y. A hydrogen-ferric ion rebalance cell operating at low hydrogen concentrations for capacity restoration of iron-chromium redox flow batteries. J. Power Sources 2017, 352, 77–82. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, X.; An, L.; Wei, L.; Zhao, T. A high-performance flow-field structured iron-chromium redox flow battery. J. Power Sources 2016, 324, 738–744. [Google Scholar] [CrossRef]
- Perry, M.L.; Rodby, K.E.; Brushett, F. Untapped potential: The need and opportunity for high-voltage aqueous redox flow batteries. ACS Energy Lett. 2022, 7, 659–667. [Google Scholar] [CrossRef]
- Hiremath, M.; Derendorf, K.; Vogt, T. Comparative life cycle assessment of battery storage systems for stationary applications. Environ. Sci. Technol. 2015, 49, 4825–4833. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Vo, D.-V.N.; Chelliapan, S.; Joo, S.-W.; Vasseghian, Y. Latest eco-friendly avenues on hydrogen production towards a circular bioeconomy: Currents challenges, innovative insights, and future perspectives. Renew. Sustain. Energy Rev. 2022, 168, 112916. [Google Scholar] [CrossRef]
- Wang, W.; Kim, S.; Chen, B.; Nie, Z.; Zhang, J.; Xia, G.-G.; Li, L.; Yang, Z.J.E.; Science, E. A new redox flow battery using Fe/V redox couples in chloride supporting electrolyte. Energy Environ. Sci. 2011, 4, 4068–4073. [Google Scholar] [CrossRef]
- Bray, K.L.; Conover, D.R.; Kintner-Meyer, M.C.; Viswanathan, V.; Ferreira, S.; Rose, D.; Schoenwald, D. Protocol for Uniformly Measuring and Expressing the Performance of Energy Storage Systems; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2012. [Google Scholar]
- Michaels, K.; Hall, G. Cost Projections for Redox Energy Storage Systems. U.S. Patent No, NASA-CR-165260, 1 February 1980. [Google Scholar]
- Tomazic, G.; Skyllas-Kazacos, M. Redox flow batteries. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 309–336. [Google Scholar]
- Fernandez-Marchante, C.M.; Millán, M.; Medina-Santos, J.I.; Lobato, J.J. Environmental and preliminary cost assessments of redox flow batteries for renewable energy storage. Energy Technol. 2020, 8, 1900914. [Google Scholar] [CrossRef]
- Arbabzadeh, M.; Johnson, J.X.; De Kleine, R.; Keoleian, G.A. Vanadium redox flow batteries to reach greenhouse gas emissions targets in an off-grid configuration. Appl. Energy 2015, 146, 397–408. [Google Scholar] [CrossRef]
- Rydh, C.J. Environmental assessment of vanadium redox and lead-acid batteries for stationary energy storage. J. Power Sources 1999, 80, 21–29. [Google Scholar] [CrossRef]
- Nikiforidis, G.; Berlouis, L.; Hall, D.; Hodgson, D. Impact of electrolyte composition on the performance of the zinc–cerium redox flow battery system. J. Power Sources 2013, 243, 691–698. [Google Scholar] [CrossRef]







| Technology | Life Cycle at 80% Depth of Discharge (DoD) | Efficiency (%) | Specific Energy (Wh/L) | Energy Density (W/L) | Advantages | Limitations | Applications | Capital Cost per Unit Energy (USD/kw h–Output) |
|---|---|---|---|---|---|---|---|---|
| Flow battery | 2000–20,000 | 65–85 | 40 | 10–50 |
| High maintenance
|
| 110–2000 |
| Lead-acid batteries | 300–3000 | 70–90 | 35–40 | 80–90 |
|
|
| 350–1500 |
| Ni-Cd batteries | 3000 | 80 | 40–60 | 50–150 |
|
|
| 800–3000 |
| Lithium-ion batteries | 3000 | 75–90 | 100–265 | 250–693 |
|
|
| 850–5000 |
| Na-S batteries | 4500 | 89 | 150–300 | 10,000 |
|
|
| NA |
| Hydrogen battery (fuel cell) | 20,000 | 20–66 | 300–300 | 500< |
|
|
| NA |
| Membrane Property | |||||||
|---|---|---|---|---|---|---|---|
| Membrane (Commercial Name) | Mechanical Property | Vanadium Cross-Over | Ionic Conductivity σ (mS·cm−1) | Area Resistance (Ω·cm2) | Ion Selectivity (S·s · cm−3·106) | Swelling Property | |
| Peak Stress Loss (%) | Diffusion Coefficients of VO2+ Ions (cm2·s−1·10−7) | Dimensional Changes (Volume Changes) (%) | Mass Changes (%) | ||||
| N212 | 20 | 0.11 | 70 ± 6 | 0.47 | 1.0 | 8 ± 4 | 6 ± 0.2 |
| FS-930 | 25 | 0.18 | 84 ± 8 | 0.54 | 0.3 | 3 ± 1 | 3 ± 1 |
| DF | 18 | 0.092 | 60 ± 1 | 0.48 | 1.4 | 6 ± 3 | 4 ± 2 |
| VAN | 40 | 1.62 | 0.54 ± 0.01 | 1.23 | 0.1 | 2 ± 1 | 101 ± 6 |
| AMV | 6 | 0.0025 | 3.9 ± 0.0 | 2.08 | 21.8 | 2 ± 2 | 9 ± 1 |
| CMV | 4 | 0.035 | 4.6 ± 0.4 | 0.62 | 5.5 | 0 ± 1 | 1 ± 1 |
| AHA | 32 | 0.032 | 2.4 ± 0.2 | 2.39 | 2.8 | 7 ± 1 | 8 ± 2 |
| FAB-450 | 7 | 0.070 | 38 ± 3 | 0.59 | 1.6 | 42 ± 8 | 57 ± 3 |
| Electrode Material | Thickness | Electrode Polarity | Flow Battery System | Refs. |
|---|---|---|---|---|
| Carbon-Based Electrodes | ||||
| Carbon felt GFA-type | 8 mm | +ve | Zinc–cerium | [175] |
| Carbon polymer binders: PVA, PVDF, HDPE | 6 mm (substrate) | −ve | Zinc–cerium | [175,176] |
| Graphite felt | NA | +ve & −ve | All–vanadium | [177] |
| PAN-based graphite felt | 5 mm | +ve | Bromine polysulfide | [178] |
| Cobalt coated PAN-based graphite felt | 5 mm | −ve | Bromine polysulfide | [178] |
| 70 ppi reticulated vitreous carbon | 1.5 mm | +ve | Soluble lead acid | [179] |
| Graphite felt bonded electrode assembly with nonconducting plastic substrate | NA | +ve & −ve | All–vanadium | [180] |
| Porous graphite | 2 mm | +ve & −ve | Zinc–chlorine | [181] |
| Carbon felt CH type | 2.8 mm | +ve & −ve | Zinc–bromine | [182] |
| Cylindrical bed of carbon particles | 2.5 mm | +ve & −ve | Bromine polysulfide | [148] |
| Metallic electrodes | ||||
| Nickel foam | 2.5 mm | −ve | Bromine polysulfide | [183,184] |
| Cadmium-plated copper | NA | −ve | Zinc–nickel and Zinc–air | [185] |
| Sintered nickel hydroxide | NA | −ve | Zinc–nickel | [183,186] |
| 40 ppi nickel foam | 40 ppi nickel foam | 40 ppi nickel foam | 40 ppi nickel foam | [179] |
| Three-dimensional platinised titanium mesh stack (4 meshes, 70 g Pt m-2 loading, 3.5 μm thick) | 4 × 2.5 mm | +ve | Zinc-cerium | [175] |
| Electrode Reactions | Catalysts |
|---|---|
| VO2+ + 2H+ + e− ⇄ VO2++ H2O | Mn3O4/carbon fiber ZrO2 Bi2O3 Nanorod Nb2O5 Ir-modification of carbon felt. WO3 PbO2 |
| V3+ + e− ⇄ V2+ | Mn3O4/carbon fiber ZrO2 Bi2O3 Nanorod Nb2O5 TiC |
| Cr3+ + e− ⇄ Cr2+ | Noble catalysts |
| Ce4+ + e− ⇄ Ce3+ | Platinized titanium |
| Cl2 + 2e− ⇄ 2Cl− | RuO2 |
| O2 + 4H+ + 4e− ⇄ 2H2O | Pt/Ir mixed oxide |
| Techniques | Function |
|---|---|
| Electrochemical Techniques | |
| Galvanostatic charge–discharge (GCD) | Analyzing flow battery performance by applying a constant current during charge and discharge processes |
| Cyclic voltammetry (CV) | Evaluating current response to a linear voltage sweep |
| Current interrupt (CI) | Relating transient voltage response to cell parameters from current interruption events |
| Electrochemical impedance spectroscopy (EIS) | Measuring the impedance of an electrochemical system over a range of frequencies |
| Rotating disk electrode (RDE) | Studying reaction mechanism behavior and mass transport characteristics |
| Ultraviolet–visible (UV–Vis) spectroscopy | Quantitatively measuring the number of ionic species present in the electrolyte |
| Galvanostatic intermittent titration technique (GITT) | Determining the diffusion coefficient by applying short current pulses and analyzing the potential response |
| Potentiostatic intermittent titration technique (PITT) | Determining the diffusion coefficient by applying short potential pulses and analyzing current response |
| Charge pulse relaxation (CPR) | Determining the diffusion coefficient by measuring the relaxation time of a charge pulse |
| Potential distribution (PD) | Examining the potential distribution of half cells and bipolar plates in a multi-cell stack |
| Nuclear magnetic resonance (NMR) spectroscopy | Observing the migration of cations or anions |
| Electron spin resonance (ESR) spectroscopy | Studying rotational diffusion for various materials containing organic free radicals or transition metals |
| Physical and Spectroscopic | |
| Thermal visualization of flow distribution | Studying and visualizing electrolyte flow patterns and distribution in flow battery systems |
| Optical visualization of flow distribution | Visualizing flow patterns and distribution using optical techniques |
| Scanning electron microscopy (SEM) | Characterizing materials on micro- and nanoscale resolutions |
| Transmission electron microscopy (TEM) | Studying the structure and properties of materials at the nanoscale |
| Energy dispersive X-ray spectroscopy (EDS) | Performing elemental analysis |
| Raman spectroscopy | Characterizing molecular vibrations |
| Brunauer–Emmett–Teller (BET) | Examining the surface area of a material |
| X-ray diffraction (XRD) analysis | Studying the crystallographic structure of materials |
| Pair distribution function (PDF) | Extracting structural information from amorphous or disordered systems |
| X-ray absorption spectroscopy (XAS) | Probing the electronic and atomic structure of materials |
| X-ray photoelectron spectroscopy (XPS) | Analyzing surface composition and chemical states |
| X-ray tomographic microscopy (XTM) | Visualizing the 3D microstructure of samples |
| X-ray fluorescence spectroscopy (XRF) | Determining the elemental composition of a sample |
| Fourier transform infrared spectroscopy (FTIR) | Identifying molecular bonds and structures |
| Atomic force microscopy (AFM) | Imaging surfaces at the atomic and molecular scale |
| Secondary ion mass spectrometry (SIMS) | Analyzing surface composition and depth profiling of materials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olabi, A.G.; Allam, M.A.; Abdelkareem, M.A.; Deepa, T.D.; Alami, A.H.; Abbas, Q.; Alkhalidi, A.; Sayed, E.T. Redox Flow Batteries: Recent Development in Main Components, Emerging Technologies, Diagnostic Techniques, Large-Scale Applications, and Challenges and Barriers. Batteries 2023, 9, 409. https://doi.org/10.3390/batteries9080409
Olabi AG, Allam MA, Abdelkareem MA, Deepa TD, Alami AH, Abbas Q, Alkhalidi A, Sayed ET. Redox Flow Batteries: Recent Development in Main Components, Emerging Technologies, Diagnostic Techniques, Large-Scale Applications, and Challenges and Barriers. Batteries. 2023; 9(8):409. https://doi.org/10.3390/batteries9080409
Chicago/Turabian StyleOlabi, Abdul Ghani, Mohamed Adel Allam, Mohammad Ali Abdelkareem, T. D. Deepa, Abdul Hai Alami, Qaisar Abbas, Ammar Alkhalidi, and Enas Taha Sayed. 2023. "Redox Flow Batteries: Recent Development in Main Components, Emerging Technologies, Diagnostic Techniques, Large-Scale Applications, and Challenges and Barriers" Batteries 9, no. 8: 409. https://doi.org/10.3390/batteries9080409