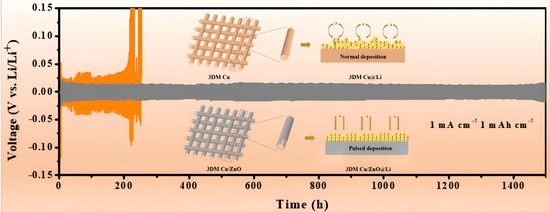
Pulsed Current Constructs 3DM Cu/ZnO Current Collector Composite Anode for Free-Dendritic Lithium Metal Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.1.1. Fabrication of 3DM Cu/ZnO Current Collector
2.1.2. Fabrication of 3DM Cu@Li, Cu/ZnO@Li-N, and Cu/ZnO@Li-P Composite Anodes
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results
3.1. Material Characterization
3.2. Deposition Morphology
3.3. Electrochemical Performance Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.X.; Bonakdarpour, A.; Stoševski, I.; Fang, B.; Wilkinson, D.P. Modification of Cu Current Collectors for Lithium Metal Batteries—A Review. Prog. Mater. Sci. 2022, 130, 100996. [Google Scholar] [CrossRef]
- Lyu, T.Y.; Luo, F.Q.; Wang, D.C.; Bu, L.Z.; Tao, L.; Zheng, Z.F. Carbon/Lithium Composite Anode for Advanced Lithium Metal Batteries: Design, Progress, In Situ Characterization, and Perspectives. Adv. Energy Mater. 2022, 12, 2201493. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Liu, B.; Shen, Y.H.; Wu, J.K.; Zhao, Z.Q.; Zhong, C.; Hu, W.B. Confronting the Challenges in Lithium Anodes for Lithium Metal Batteries. Adv. Sci. 2021, 8, e2101111. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, X.L.; Ji, X.; Chen, J.; Hou, S.; Wang, C.S. High-Energy Li Metal Battery with Lithiated Host. Joule 2019, 3, 732–744. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Shi, H.; Yang, S.T.; Cai, Z.J.; Lu, H.Q.; Jia, L.T.; Hu, M.Z.; He, H.; Zhou, K.B. Oxygen Vacancy-Enriched Co3O4 as Lithiophilic Medium for Ultra-Stable Anode of Lithium Metal Batteries. J. Alloys Compd. 2021, 888, 161553. [Google Scholar] [CrossRef]
- Bao, W.Z.; Wang, R.H.; Qian, C.F.; Li, M.H.; Sun, K.W.; Yu, F.; Liu, H.; Guo, C.; Li, J.F. Photoassisted High-Performance Lithium Anode Enabled by Oriented Crystal Planes. ACS Nano 2022, 6, 17454–17465. [Google Scholar] [CrossRef]
- Cho, S.J.; Kim, D.Y.; Lee, J.I.; Kang, J.S.; Lee, H.; Kim, G.; Seo, D.H.; Park, S. Highly Reversible Lithium Host Materials for High-Energy-Density Anode-Free Lithium Metal Batteries. Adv. Funct. Mater. 2022, 32, 2208629. [Google Scholar] [CrossRef]
- Liang, P.; Sun, H.; Huang, C.L.; Zhu, G.Z.; Tai, H.C.; Li, J.C.; Wang, F.F.; Wang, Y.; Huang, C.J.; Jiang, S.K.; et al. A Nonflammable High-Voltage 4.7 V Anode-Free Lithium Battery. Adv. Mater. 2022, 34, e2207361. [Google Scholar] [CrossRef]
- Zhang, P.P.; Yang, S.; Xie, H.G.; Li, Y.; Wang, F.X.; Gao, M.M.; Guo, K.; Wang, R.H.; Lu, X. Advanced Three-Dimensional Microelectrode Architecture Design for High-Performance On-Chip Micro-Supercapacitors. ACS Nano 2022, 16, 17593–17612. [Google Scholar] [CrossRef]
- Sun, J.H.; Peng, J.Y.; Ring, T.; Whittaker-Brooks, J.; Zhu, J.; Fraggedakis, D.; Niu, J.; Gao, T.; Wang, F. Lithium Deposition Mechanism on Si and Cu Substrates in the Carbonate Electrolyte. Energ. Environ. Sci. 2022, 15, 5284–5299. [Google Scholar] [CrossRef]
- Chen, X.R.; Li, B.Q.; Zhu, C.; Zhang, R.; Cheng, X.B.; Huang, J.Q.; Zhang, Q. A Coaxial-Interweaved Hybrid Lithium Metal Anode for Long-Lifespan Lithium Metal Batteries. Adv. Energy Mater. 2019, 9, 1901932. [Google Scholar] [CrossRef]
- Sun, X.W.; Zhang, X.Y.; Ma, Q.T.; Guan, X.Z.; Wang, W.; Luo, J.Y. Revisiting the Electroplating Process for Lithium-Metal Anodes for Lithium-Metal Batteries. Angew Chem. Int. Ed. Engl. 2020, 59, 6665–6674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Cheng, Y.; Chen, H.B.; Wang, Y.; Chen, Q.; Hou, G.Y.; Wen, M.; Tang, Y.P. MoP Quantum Dot-Modified N, P-Carbon Nanotubes as a Multifunctional Separator Coating for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Inter. 2022, 14, 16289–16299. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.T.; Zheng, Y.; Or, T.; Park, H.W.; Gao, R.; Park, M.; Ma, Q.Y.; Luo, D.; Yu, A.P.; Chen, Z.W. Advanced Material Engineering to Tailor Nucleation and Growth towards Uniform Deposition for Anode-Less Lithium Metal Batteries. Small 2022, 18, e2205233. [Google Scholar] [CrossRef]
- Deng, W.; Yin, X.; Bao, W.; Zhou, X.F.; Hu, Z.Y.; He, B.Y.; Qiu, B.; Meng, Y.S.; Liu, Z.P. Quantification of Reversible and Irreversible Lithium in Practical Lithium-Metal Batteries. Nat. Energy 2022, 7, 1031–1041. [Google Scholar] [CrossRef]
- Luo, C.; Hu, H.; Zhang, T.; Wen, S.J.; Wang, R.; An, Y.N.; Chi, S.S.; Wang, J.; Wang, C.Y.; Chang, J.; et al. Roll-To-Roll Fabrication of Zero-Volume-Expansion Lithium-Composite Anodes to Realize High-Energy-Density Flexible and Stable Lithium-Metal Batteries. Adv. Mater. 2022, 34, e2205677. [Google Scholar] [CrossRef]
- Wu, Q.P.; Zheng, Y.J.; Guan, X.; Xu, J.; Cao, F.H.; Li, C.L. Dynamical SEI Reinforced by Open-Architecture MOF Film with Stereoscopic Lithiophilic Sites for High-Performance Lithium-Metal Batteries. Adv. Funct. Mater. 2021, 31, 2101034. [Google Scholar] [CrossRef]
- Li, L.L.; Li, S.Y.; Lu, Y.Y. Suppression of Dendritic Lithium Growth in Lithium Metal-Based Batteries. Chem. Commun. 2018, 54, 6648–6661. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, H.M.; Liu, X.; Zhou, C.; Li, G.R.; Liu, S.; Gao, X.P. A Dimensionally Stable Lithium Alloy Based Composite Electrode for Lithium Metal Batteries. Chem. Eng. J. 2022, 450, 138074. [Google Scholar] [CrossRef]
- Wang, H.S.; Lin, D.C.; Xie, J.; Liu, Y.Y.; Chen, H.; Li, Y.B.; Xu, J.W.; Zhou, G.M.; Zhang, Z.; Pei, A.; et al. An Interconnected Channel-Like Framework as Host for Lithium Metal Composite Anodes. Adv. Energy Mater. 2019, 9, 1802720. [Google Scholar] [CrossRef]
- Zhang, J.L.; Chen, H.B.; Wen, M.; Shen, K.; Chen, Q.; Hou, G.Y.; Tang, Y.P. Lithiophilic 3D Copper-Based Magnetic Current Collector for Lithium-Free Anode to Realize Deep Lithium Deposition. Adv. Funct. Mater. 2021, 32, 2110110. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yuan, B.Y.; Sun, C.; Lu, Y.H.; Lin, X.P.; Chen, M.H.; Xie, Y.S.; Zhang, S.Q.; Lai, C. Ultralow-Expansion Lithium Metal Composite Anode via Gradient Framework Design. Adv. Funct. Mater. 2022, 32, 2202771. [Google Scholar] [CrossRef]
- Qian, J.F.; Adams, B.D.; Zheng, J.M.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.C.; Hu, J.Z.; Zhang, J.G. Anode-Free Rechargeable Lithium Metal Batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Lin, L.D.; Qin, K.; Hu, Y.S.; Li, H.; Huang, X.J.; Suo, L.M.; Chen, L.Q. A Better Choice to Achieve High Volumetric Energy Density: Anode-Free Lithium-Metal Batteries. Adv. Mater. 2022, 34, e2110323. [Google Scholar] [CrossRef]
- Lai, G.M.; Jiao, J.Y.; Fang, C.; Jiang, Y.; Sheng, L.Y.; Xu, B.; Ouyang, C.Y.; Zheng, J.X. The Mechanism of Li Deposition on the Cu Substrates in the Anode-Free Li Metal Batteries. Small 2022, 19, e2205416. [Google Scholar] [CrossRef]
- Zhang, J.L.; Chen, H.B.; Fan, B.F.; Shan, H.P.; Chen, Q.; Jiang, C.H.; Hou, G.Y.; Tang, Y.P. Study on the Relationship between Crystal Plane Orientation and Strength of Electrolytic Copper Foil. J. Alloys Compd. 2021, 884, 161044. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, S.P.; Lu, Y.Y. 3D Porous Cu Current Collector/Li-Metal Composite Anode for Stable Lithium-Metal Batteries. Adv. Funct. Mater. 2017, 27, 1606422. [Google Scholar] [CrossRef]
- Li, D.D.; Xie, C.; Gao, Y.; Hu, H.; Wang, L.; Zheng, Z.J. Inverted Anode Structure for Long-Life Lithium Metal Batteries. Adv. Energy Mater. 2022, 12, 2200584. [Google Scholar] [CrossRef]
- Ryou, M.H.; Kim, S.H.; Kim, S.W.; Lee, S.Y. A Microgrid-Patterned Silicon Electrode as an Electroactive Lithium Host. Energ. Environ. Sci. 2022, 15, 2581–2590. [Google Scholar] [CrossRef]
- Lee, H.; Song, J.C.; Kim, Y.J.; Park, J.K.; Kim, H.T. Structural Modulation of Lithium Metal-Electrolyte Interface with Three-Dimensional Metallic Interlayer for High-Performance Lithium Metal Matteries. Sci. Rep. 2016, 6, 30830. [Google Scholar] [CrossRef]
- Chu, C.X.; Li, R.; Cai, F.P.; Bai, Z.C.; Wang, Y.X.; Xu, X.; Wang, N.N.; Yang, J.; Dou, S.X. Recent Advanced Skeletons in Sodium Metal Anodes. Energ. Environ. Sci. 2021, 14, 4318–4340. [Google Scholar] [CrossRef]
- Sun, C.Y.; Gao, L.; Yang, Y.H.; Yan, Z.C.; Zhang, D.M.; Bian, X.F. Ultrafast Microwave-Induced Synthesis of Lithiophilic Oxides Modified 3D Porous Mesh Skeleton for High-Stability Li-Metal Anode. Chem. Eng. J. 2023, 452, 139407. [Google Scholar] [CrossRef]
- Louli, A.J.; Eldesoky, A.; de Gooyer, J.; Coon, M.; Aiken, C.P.; Simunovic, Z.; Metzger, M.; Dahn, J.R. Different Positive Electrodes for Anode-Free Lithium Metal Cells. J. Electrochem. Soc. 2022, 169, 040517. [Google Scholar] [CrossRef]
- Liu, Y.H.; Sun, J.M.; Hu, X.Q.; Li, Y.F.; Du, H.F.; Wang, K.; Du, Z.Z.; Gong, X.; Ai, W.; Huang, W. Lithiophilic Sites Dependency of Lithium Deposition in Li Metal Host Anodes. Nano Energy 2022, 94, 106883. [Google Scholar] [CrossRef]
- Zhan, Y.X.; Shi, P.; Ma, X.X.; Jin, C.B.; Zhang, Q.K.; Yang, S.J.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q. Failure Mechanism of Lithiophilic Sites in Composite Lithium Metal Anode under Practical Conditions. Adv. Energy Mater. 2021, 12, 2103291. [Google Scholar] [CrossRef]
- Shen, K.; Wang, Z.; Bi, X.X.; Ying, Y.; Zhang, D.; Jin, C.B.; Hou, G.Y.; Cao, H.Z.; Wu, L.K.; Zheng, G.Q.; et al. Magnetic Field-Suppressed Lithium Dendrite Growth for Stable Lithium-Metal Batteries. Adv. Energy Mater. 2019, 9, 1900260. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhou, Z.K.; Wang, Y.; Chen, Q.; Hou, G.Y.; Tang, Y.P. Ultrasonic-Assisted Enhancement of Lithium-Oxygen Battery. Nano Energy 2022, 102, 107655. [Google Scholar] [CrossRef]
- Zhang, X.D.; Liu, T.Y.; Liu, C.; Zheng, D.S.; Huang, J.M.; Liu, Q.W.; Yuan, W.W.; Yin, Y.; Huang, L.R.; Xu, M.; et al. Asymmetric Low-Frequency Pulsed Strategy Enables Ultralong CO2 Reduction Stability and Controllable Product Selectivity. J. Am. Chem. Soc. 2023, 145, 2195–2206. [Google Scholar] [CrossRef]
- Li, S.N.; Zhang, H.; Ruan, H.C.; Cheng, Z.H.; Yao, Y.G.; Zhuge, F.W.; Zhai, T.Y. Programmable Nucleation and Growth of Ultrathin Tellurium Nanowires via a Pulsed Physical Vapor Deposition Design. Adv. Funct. Mater. 2022, 33, 2211527. [Google Scholar] [CrossRef]
- Xiao, T.; Zhou, Z.K.; Cao, H.; Zhang, J.L.; Chen, Q.; Hou, G.Y.; Wen, M.; Tang, Y.P. Pulse Current Charging Strategy towards High Performance of Lithium-Oxygen Batteries. Surf. Interfaces 2021, 24, 101106. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhou, Z.K.; Wang, Y.; Chen, Q.; Hou, G.Y.; Tang, Y.P. Pulsed Current Boosts the Stability of the Lithium Metal Anode and the Improvement of Lithium-Oxygen Battery Performance. ACS Appl. Mater. Inter. 2022, 14, 50414–50423. [Google Scholar] [CrossRef]
- Liu, F.; Xu, R.; Wu, Y.C.; Boyle, D.T.; Yang, A.K.; Xu, J.W.; Zhu, Y.Y.; Ye, Y.S.; Yu, Z.A.; Zhang, Z.W.; et al. Dynamic Spatial Progression of Isolated Lithium During Battery Operations. Nature 2021, 600, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, K.; Han, Y.; Sun, Z.H.; Zhang, H.T.; Xu, L.Q.; Ma, Y.F.; Chen, Y.S. A Nitrogen-Doped-Carbon/ZnO Modified Cu Foam Current Collector for High-Performance Li Metal Batteries. J. Mater. Chem. A 2019, 7, 5712. [Google Scholar] [CrossRef]
- Sun, C.Z.; Li, Y.P.; Jin, J.; Yang, J.H.; Wen, Z.Y. ZnO Nanoarray-Modified Nickel Foam as a Lithiophilic Skeleton to Regulate Lithium Deposition for Lithium-Metal Batteries. J. Mater. Chem. A 2019, 7, 7752. [Google Scholar] [CrossRef]
- Shen, Y.X.; Pu, Z.Y.; Zhang, Y.R.; Chen, Y.; Zhang, H.; Wang, N.T.; Qiu, H.L.; Li, Y.M. MXene/ZnO Flexible Freestanding Film as a Dendrite-Free Support in Linthium Metal Batteries. J. Mater. Chem. A 2022, 10, 17199. [Google Scholar] [CrossRef]
- Ni, C.K.; Mao, J.T.; Cheng, Z.L.; Pan, P.; Jiang, L.Y.; Wang, Z.X.; Zhang, M.M.; Zhang, Y.R.; Xing, Y.S.; Zeng, Y.; et al. Si/ZnO Framework: 3D Lithiophilic Structure for Dendrite-Free Lithium Metal Batteries. J. Alloys Compd. 2021, 25, 876. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Chen, Q.; Wang, Y.; Hou, G.; Zhang, J.; Tang, Y. Pulsed Current Constructs 3DM Cu/ZnO Current Collector Composite Anode for Free-Dendritic Lithium Metal Batteries. Batteries 2023, 9, 188. https://doi.org/10.3390/batteries9030188
Zhou Z, Chen Q, Wang Y, Hou G, Zhang J, Tang Y. Pulsed Current Constructs 3DM Cu/ZnO Current Collector Composite Anode for Free-Dendritic Lithium Metal Batteries. Batteries. 2023; 9(3):188. https://doi.org/10.3390/batteries9030188
Chicago/Turabian StyleZhou, Zhenkai, Qiang Chen, Yang Wang, Guangya Hou, Jianli Zhang, and Yiping Tang. 2023. "Pulsed Current Constructs 3DM Cu/ZnO Current Collector Composite Anode for Free-Dendritic Lithium Metal Batteries" Batteries 9, no. 3: 188. https://doi.org/10.3390/batteries9030188