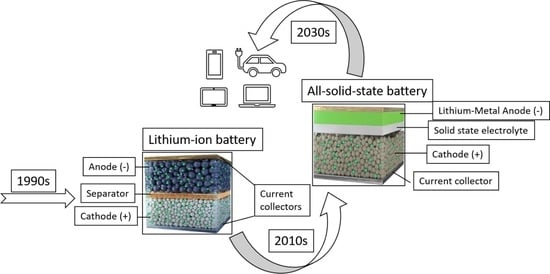
Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use
Abstract
:1. Introduction
1.1. Theoretical Aspects of Li-Ion Battery Technology
1.1.1. Key Parameters of LIB Development
1.1.2. Solid Electrolyte Interface (SEI) Formation
1.1.3. Safety Concerns
1.1.4. Cathode Materials Applicable in Lithium-Ion Batteries
1.1.5. Anode Materials for Lithium-Ion Batteries
- Lithium-based anodes
- Graphene-based anodes
- Graphite-based anodes
- Ti-based oxides anode materials
- Silicon-based anode materials
1.2. Challenges Associated with All-Solid-State Batteries
Solid-State Electrolytes
- − Good ionic conductivity and negligible electronic conductivity with a wide working temperature range.
- − A chemical potential range between that of the Li metal anode and the corresponding cathode.
- − Negligible grain boundary resistance and interface resistance at the electrode-electrolyte interface.
- − For high temperature operation, thermal and mechanical properties, e.g., the thermal expansion coefficient, match those of the anode and cathode.
- − High chemical stability, in connection with that of the metal anode and high voltage cathode.
- − Low cost, environmental safety, easy to scale up and prepare.
- Solid inorganic electrolytes
- − High fragility.
- − Poor contact with the electrode and inferior interfacial charge transport leads to high impedance.
- − Dendrite growth and propagation through grain boundaries, especially at lower current densities
- − High cost and poor environmental stability.
- Solid Polymer Electrolytes
- Solid hybrid electrolytes
2. Assessment of All-Solid-State Batteries Patents
2.1. Overview of Patents on Solid Inorganic Electrolytes
2.2. Solid Polymer Electrolytes
2.3. Overview of Hybrid (Ceramic/Polymer) Solid Electrolytes
3. Global Overview of ASSB-Producing Solid-State Battery Companies
- Companies not providing detailed information on ASSBs
- Suppliers and manufacturers of materials and components for ASSBs
- Companies applying hybrid technologies
- Companies cooperating with other firms and investing in the development of ASSBs
- Companies with their own technology that lack a prototype or device
- Companies conducting research on the topic that have not openly disclosed any results
- Companies that have published information on ASSB prototypes
- Companies with an ASSB product sold commercially
4. Conclusions, Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.researchandmarkets.com/reports/4396452/lithium-ion-battery-market-size-share-and-trends (accessed on 15 November 2022).
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2015, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C.S. A Novel Aluminum–Graphite Dual-Ion Battery. Adv. Energy Mater. 2016, 6, 1502588. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H.-M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M.; et al. Lithium-ion batteries—Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, C.; Ou, T.; Zhang, S.; Li, L.; Ji, X. Constructing advanced electrode materials for low-temperature lithium-ion batteries: A review. Energy Rep. 2022, 8, 4525–4534. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-Ion batteries at extreme temperatures. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Wang, D.; Zhang, J. Fundamentals and Challenges of Lithium Ion Batteries at Temperatures between −40 and 60 °C. Adv. Energy Mater. 2020, 10, 1904152. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x < −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Yu, W.; Ou, G.; Qi, L.; Wu, H. Textured LiFePO4 Bulk with Enhanced Electrical Conductivity. J. Am. Ceram. Soc. 2016, 99, 3214–3216. [Google Scholar] [CrossRef]
- Chen, S.-P.; Lv, D.; Chen, J.; Zhang, Y.-H.; Shi, F.-N. Review on Defects and Modification Methods of LiFePO4 Cathode Material for Lithium-Ion Batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Sharma, N.; Yu, D.H.; Zhu, Y.; Wu, Y.; Peterson, V.K. In operando neutron diffraction study of the temperature and current rate-dependent phase evolution of LiFePO4 in a commercial battery. J. Power Sources 2017, 342, 562–569. [Google Scholar] [CrossRef]
- Liao, L.; Cheng, X.; Ma, Y.; Zuo, P.; Fang, W.; Yin, G.; Gao, Y. Fluoroethylene carbonate as electrolyte additive to improve low temperature performance of LiFePO4 electrode. Electrochim. Acta 2013, 87, 466–472. [Google Scholar] [CrossRef]
- Ma, Q.; Zeng, X.-X.; Yue, J.; Yin, Y.-X.; Zuo, T.-T.; Liang, J.-Y.; Deng, Q.; Wu, X.-W.; Guo, Y.-G. Viscoelastic and Nonflammable Interface Design–Enabled Dendrite-Free and Safe Solid Lithium Metal Batteries. Adv. Energy Mater. 2019, 9, 1803854. [Google Scholar] [CrossRef]
- Guohua, T. Nonequilibrium Electron-Coupled Lithium Ion Diffusion in LiFePO4: Nonadiabatic Dynamics with Multistate Trajectory Approach. J. Phys. Chem. C 2016, 120, 6938–6952. [Google Scholar] [CrossRef]
- Tu, W.; Xia, P.; Li, J.; Zeng, L.; Xu, M.; Xing, L.; Zhang, L.; Yu, L.; Fan, W.; Li, W. Terthiophene as electrolyte additive for stabilizing lithium nickel manganese oxide cathode for high energy density lithium-ion batteries. Electrochim. Acta 2016, 208, 251–259. [Google Scholar] [CrossRef]
- Visco, S.; Katz, B.; Nimon, Y.; De Jonghe, L. Protected Active Metal Electrode and Battery Cell Structures with Non-Aqueous Interlayer Architecture. U.S. Patent US7282295B2, 28 June 2007. [Google Scholar]
- Abraham, K.M.; Jiang, J. A Polymer Electrolyte—Based Rechargeable Lithium/Oxygen Battery. Electrochem. Soc. 1996, 143, 1. [Google Scholar] [CrossRef]
- Laoire, C.Ó.; Mukerjee, S.; Plichta, E.J.; Hendrickson, M.A.; Abraham, K.M. Rechargeable Lithium/TEGDME-LiPF6ÕO2 Battery. J. Electrochem. Soc. 2011, 158, A302–A308. [Google Scholar] [CrossRef]
- Herbert, D.; Ulam, Z.U.S. Electric Dry Cells and Storage Batteries. Patent 3043896, 7 October 1962. [Google Scholar]
- Bruce, P.G.; Hardwick, L.J.; Abraham, K.M. Lithium-air and lithium-sulfur batteries. MRS Bull. 2011, 36, 506–512. [Google Scholar] [CrossRef]
- Cai, K.; Wang, T.; Wang, Z.; Wang, J.; Li, L.; Yao, C.; Lang, X. A cocklebur-like sulfur host with the TiO2-VOx heterostructure efficiently implementing one-step adsorption-diffusion-conversion towards long-life Li–S batteries. Compos. Part B Eng. 2023, 249, 110410. [Google Scholar] [CrossRef]
- Manthiram, A. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N.A. Perspective on the Sustainability of Cathode Materials used in Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2102028. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Chen, Z.; Xu, K.; Lu, J. New Concepts in Electrolytes. Chem. Rev. 2020, 120, 6783–6819. [Google Scholar] [CrossRef]
- Zu, C.; Yu, H.; Li, H. Enabling the thermal stability of solid electrolyte interphase in Li-ion battery. InfoMat 2021, 3, 648–661. [Google Scholar] [CrossRef]
- Pinson, M.B.; Bazant, M.Z.J. Theory of SEI Formation in Rechargeable Batteries: Capacity Fade, Accelerated Aging and Lifetime Prediction. J. Electrochem. Soc. 2013, 160, A243–A250. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, P.; Ren, D.; Yang, M.; Rui, X.; Jin, C.; Shen, T.; Han, X.; Zheng, Y.; Lu, L.; et al. A mechanistic calendar aging model of lithium-ion battery considering solid electrolyte interface growth. Int. J. Energy Res. 2022, 46, 15521–15534. [Google Scholar] [CrossRef]
- Hamidah, N.L.; Wang, F.M.; Nugroho, G. The understanding of solid electrolyte interface (SEI) formation and mechanism as the effect of flouro-o-phenylenedimaleimaide (F-MI) additive on lithium-ion battery. Surf. Interface Anal. 2019, 51, 345–352. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xu, C.; et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Hou, J.; Lu, L.; Wang, L.; Ohma, A.; Ren, D.; Feng, X.; Li, Y.; Li, Y.; Ootani, I.; Han, X.; et al. Thermal runaway of Lithium-ion batteries employing LiN(SO2F)2-based concentrated electrolytes. Nat Commun. 2020, 11, 5100. [Google Scholar] [CrossRef]
- Jia, H.; Xu, W. Nonflammable nonaqueous electrolytes for lithium batteries. Curr. Opin. Electrochem. 2021, 30, 100781. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.-L.; Zhuang, M.; Gao, H.; Lu, L.; Han, X.; Chu, Z.; et al. Thermal runaway of lithium-ion batteries without internal short circuits. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Liu, X.; Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. Model-based thermal runaway prediction of lithium-ion batteries from kinetics analysis of cell components. Appl. Energy 2018, 228, 633–644. [Google Scholar] [CrossRef]
- Swiderska-Mocek, A.; Jakobczyk, P.; Rudnicka, E.; Lewandowski, A. Flammability parameters of lithium-ion battery electrolytes. J. Mol. Liq. 2020, 318, 113986. [Google Scholar] [CrossRef]
- Kawamura, T.; Kimura, A.; Egashira, M.; Okada, S.; Yamaki, J.-I. Thermal stability of alkyl carbonate mixed-solvent electrolytes for lithium ion cells. J. Power Sources 2002, 104, 260–264. [Google Scholar] [CrossRef]
- Kawamura, T.; Okada, S.; Yamaki, J.-I. Decomposition reaction of LiPF6-based electrolytes for lithium ion cells. J. Power Sources 2006, 156, 547–554. [Google Scholar] [CrossRef]
- Ping, P.; Wang, Q.; Sun, J.; Xiang, H.; Chen, C. Thermal Stabilities of Some Lithium Salts and Their Electrolyte Solutions with and Without Contact to a LiFePO4 Electrode. J. Electrochem. Soc. 2010, 157, A1170. [Google Scholar] [CrossRef]
- Sloop, S.E.; Pugh, J.K.; Wang, S.; Kerr, J.B.; Kinoshita, K. Chemical Reactivity of PF5 and LiPF6 in Ethylene Carbonate/Dimethyl Carbonate Solutions. Electrochem. Solid-State Lett. 2001, 4, A42. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; Hei, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Tian, X.; Yi, Y.; Fang, B.; Yang, P.; Wang, T.; Liu, P.; Qu, L.; Li, M.; Zhang, S. Design Strategies of Safe Electrolytes for Preventing Thermal Runaway in Lithium Ion Batteries. Chem. Mater. 2020, 32, 9821–9848. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Liu, J.; Wang, J.; Yan, W. Gas Sensing Technology for the Detection and Early Warning of Battery Thermal Runaway: A Review. Energy Fuels 2022, 36, 6038–6057. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Yuan, H.; Huang, J. Thermally Stable and Nonflammable Electrolytes for Lithium Metal Batteries: Progress and Perspectives. Small Sci. 2021, 1, 2100058. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.-B.; Jin, Z.; Zhang, R.; Wang, G.; Chen, L.-Q.; Liu, Q.-B.; Huang, J.-Q.; Zhang, Q. Recent advances in understanding dendrite growth on alkali metal anodes. Energy. Chem. 2019, 1, 100003. [Google Scholar] [CrossRef]
- Kamesui, G.; Nishikawa, K.; Matsushima, H.; Ueda, M. In Situ Observation of Cu2+ Concentration Profile during Cu Dissolution in Magnetic Field. J. Electrochem. Soc. 2021, 168, 031507. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Liu, X.; Luo, J. Dendrites in Lithium Metal Anodes: Suppression, Regulation, and Elimination. Acc. Chem. Res. 2019, 52, 3223–3232. [Google Scholar] [CrossRef]
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S.W. Sodium Metal Anodes: Emerging Solutions to Dendrite Growth. Chem. Rev. 2019, 119, 5416–5460. [Google Scholar] [CrossRef]
- Roth, E.P.; Orendorff, C.J. How Electrolytes Influence Battery Safety. Electrochem. Soc. Interface 2012, 21, 45. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on litium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [Green Version]
- Booth, S.G.; Nedoma, A.J.; Anthonisamy, N.N.; Baker, P.J.; Boston, R.; Bronstein, H.; Clarke, S.J.; Cussen, E.J.; Daramalla, V.; De Volder, M.; et al. Perspectives for next generation lithium-ion battery cathode materials. APL Mater. 2021, 9, 109201. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, X.; van Bommel, A.; Rowe, A.W.; Dahn, J.R. Coprecipitation Synthesis of NixMn1−x(OH)2 Mixed Hydroxides. Chem. Mater. A 2010, 22, 1015–1021. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Myung, S.-T.; Kim, M.-H.; Prakash, J.; Amine, K. Synthesis and Characterization of Ni[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the Microscale Core−Shell Structure as the Positive Electrode Material for Lithium Batteries. Am. Chem. Soc. 2005, 127, 13411–13418. [Google Scholar] [CrossRef]
- Hou, P.; Guo, J.; Song, D.; Zhang, J.; Zhou, E.; Zhang, L. A Novel Double-shelled LiNi0.5Co0.2Mn0.3O2 Cathode Material for Li-ion Batteries. Chem. Lett. 2012, 41, 1712–1714. [Google Scholar] [CrossRef]
- Bubulinca, C.; Sapurina, I.; Kazantseva, N.E.; Vilčáková, J.; Cheng, Q.; Sáha, P. Fabrication of a flexible binder-free lithium manganese oxide cathode for secondary Li—Ion batteries. J. Phys. Chem. Solids 2020, 137, 109222. [Google Scholar] [CrossRef]
- Bubulinca, C.; Sapurina, I.; Kazantseva, N.E.; Pechancova, V.; Saha, P. A Self-Standing Binder-Free Biomimetic Cathode Based on LMO/CNT Enhanced with Graphene and PANI for Aqueous Rechargeable Batteries. Int. J. Mol. Sci. 2022, 23, 1457. [Google Scholar] [CrossRef]
- Sapurina, I.; Bubulinca, C.; Trchová, M.; Prokeš, J.; Stejskal, J. Solid manganese dioxide as heterogeneous oxidant of aniline in the preparation of conducting polyaniline or polyaniline/manganese dioxide composites. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128298. [Google Scholar] [CrossRef]
- Shi, P.; Hou, L.-P.; Jin, C.-B.; Xiao, Y.; Yao, Y.-X.; Xie, J.; Li, B.-Q.; Zhang, X.-Q.; Zhang, Q. A successive conversion-deintercalation delithiation mechanism for practical composite lithium anodes. J. Am. Chem. Soc. 2022, 144, 212–218. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Sun, D.; Tan, Z.; Tian, X.; Ke, F.; Wu, Y.; Zhang, J. Graphene: A promising candidate for charge regulation in high-performance lithium-ion batteries. Nano Res. 2021, 14, 4370–4385. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material-fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Lou, S.; Zhao, Y.; Wang, J.; Yin, G.; Du, C.; Sun, X. Ti-Based Oxide Anode Materials for Advanced Electrochemical Energy Storage: Lithium/Sodium Ion Batteries and Hybrid Pseudocapacitors. Small 2019, 15, 1904740. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, C.; Wang, N.; Luo, L.; Chen, Y.; Li, J.; Guo, Z. Advanced composites of complex Ti-based oxides as anode materials for lithium-ion batteries. Adv. Compos. Hyb. Mat. 2018, 3, 440–459. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, L.; Liu, H.; Wang, L.; Wang, S.; Tang, Z. Mo6+–P5+ co-doped Li2ZnTi3O8 anode for Li-storage in a wide temperature range and applications in LiNi0.5Mn1.5O4/Li2ZnTi3O8 full cells. Inorg. Chem. Fron. 2021, 9, 35–43. [Google Scholar] [CrossRef]
- Sun, L.; Kong, W.; Wu, H.; Wu, Y.; Wang, D.; Zhao, F.; Jiang, K.; Li, Q.; Wang, J.; Fan, S. Mesoporous Li4Ti5O12 nanoclusters anchored on super-aligned carbon nanotubes as high-performance electrodes for lithium ion batteries. Nanoscale 2016, 8, 617–625. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, P. Insight into the effects of conductive PANI layer on Li4Ti5O12 nanofibers anode for lithium-ion batteries. Solid State Ion. 2017, 311, 52–57. [Google Scholar] [CrossRef]
- Bresser, D.; Paillard, E.; Passerini, S. Advances in Batteries for Medium and Large-Scale Energy Storage; Elsevier: Amsterdam, The Netherlands, 2014; pp. 125–211. [Google Scholar]
- He, W.; Tian, H.J.; Xin, F.X.; Han, W.Q. Scalable fabrication of micro-sized bulk porous Si from Fe–Si alloy as a high-performance anode for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 17956–17962. [Google Scholar] [CrossRef]
- Mados, E.; Harpak, N.; Levi, G.; Patolsky, E.; Peled, E.; Golodniitsky, D. Synthesis and electrochemical performance of silicon-nanowire alloy anodes. RSC Adv. 2021, 11, 26586–28593. [Google Scholar] [CrossRef]
- Wang, F.; Sun, L.; Zi, W.W.; Zhao, B.X.; Du, H.B. Solution Synthesis of Porous Silicon Particles as an Anode Material for Lithium Ion Batteries. Chem. Eur. J. 2019, 25, 9071–9077. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, M.W.; Xu, C.; Xiao, W.L.; Yamagata, H.; Xie, S.H. Effects of Sr, Ce and P on the microstructure and mechanical properties of rapidly solidified Al-7Si alloys. Mater. Charact. 2018, 140, 290. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Lee, C.-S.; Tang, Y.B. Low-Cost Metallic Anode Materials for High Performance Rechargeable Batteries. Adv. Energy Mater. 2017, 7, 1700536. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Zheng, Y.; Wang, D.; Zheng, Y.; Xie, C.; Shi, L.; Liu, Z.; Tang, Y. Unusual Size Effect in Ion and Charge Transport in Micron-sized Particulate Aluminum Anodes of Lithium-ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202208370. [Google Scholar] [CrossRef]
- Goodenough, B.J. How we made the Li-ion rechargeable battery. Nat. Electron. 2018, 1, 204. [Google Scholar] [CrossRef] [Green Version]
- 2022. Available online: https://www.quantumscape.com/resources/blog/the-advantages-of-lithium-metal-anodes/ (accessed on 25 July 2022).
- Tong, Z.; Wang, S.-B.; Liao, Y.-K.; Hu, S.-F.; Liu, R.-S. Interface Between Solid-State Electrolytes and Li-Metal Anodes: Issues, Materials, and Processing Routes. ACS Appl. Mater. Interfaces 2020, 12, 47181–47196. [Google Scholar] [CrossRef]
- Chung, H.; Kang, B. Mechanical and Thermal Failure Induced by Contact between a Li1.5Al0.5Ge1.5(PO4)3 Solid Electrolyte and Li Metal in an All Solid-State Li Cell. Chem. Mat. 2017, 29, 8611–8619. [Google Scholar] [CrossRef]
- Lou, S.; Zhang, F.; Fu, C.; Chen, M.; Ma, Y.; Yin, G.; Wang, J. Interface Issues and Challenges in All-Solid-State Batteries: Lithium, Sodium, and Beyond. Adv. Mater. 2021, 33, 2000721. [Google Scholar] [CrossRef]
- Wu, X.; Jiulin, W.; Fei, D.; Xilin, C.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Wenzel, S.; Leichtweiss, T.; Krüger, D.; Sann, J.; Janek, J. Interphase formation on lithium solid electrolytes—An in-situ approach to study interfacial reactions by photoelectron spectroscopy. Solid State Ion. 2015, 278, 98–105. [Google Scholar] [CrossRef]
- Wan, J.; Yan, H.-J.; Wen, R.; Wan, L.-J. In Situ Visualization of Electrochemical Processes in Solid-State Lithium Batteries. ACS Energy Lett. 2022, 7, 2988–3002. [Google Scholar] [CrossRef]
- Han, F.; Zhu, Y.; He, X.; Mo, Y.; Wang, C. Electrochemical Stability of Li10GeP2S12 and Li7La3Zr2O12 Solid Electrolytes. Adv. Energy Mater. 2016, 6, 1501590. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Rettenwander, D.; Wagner, R.; Reyer, A.; Bonta, M.; Cheng, L.; Doeff, M.M.; Limbeck, A.; Wilkening, M.; Amthauer, G. Interface Instability of Fe-Stabilized Li7La3Zr2O12 versus Li Metal. J. Phys. Chem. C 2018, 122, 3780–3785. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Leu, I.; Fung, K.; Hon, M.; Hsu, M.; Hsiao, Y.; Wang, M. Mechanism of the interfacial reaction between cation-deficient La0.56Li0.33TiO3 and metallic lithium at room temperature. J. Mater. Res. 2006, 23, 1813–1825. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef] [PubMed]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Goodenough, J.B. Oxide-Ion Electrolytes. Annu. Rev. Mater. Res. 2003, 33, 91–128. [Google Scholar] [CrossRef]
- Paul, P.P.; Chen, B.-R.; Langevin, A.S.; Dufek, J.E.; Weker Nelson, J.; Ko, S.J. Interfaces in all solid-state Li-metal batteries: A review on instabilities, stabilization strategies, and scalability. Energy Storage Mater. 2022, 45, 969–1001. [Google Scholar] [CrossRef]
- Sheng, O.; Jin, C.; Ding, X.; Liu, T.; Wan, Y.; Liu, Y.; Nai, J.; Wang, Y.; Liu, C.; Tao, X. A Decade of Progress on Solid-State Electrolytes for Secondary Batteries: Advances and Contributions. Adv. Funct. Mater. 2021, 31, 2100891. [Google Scholar] [CrossRef]
- Takada, K. Interfacial Nano architectonics for Solid-State Lithium Batteries. Langmuir 2013, 29, 7538–7541. [Google Scholar] [CrossRef]
- Gittleson, S.F.; El Gabaly, F. Non-Faradaic Li+ Migration and Chemical Coordination across Solid-State Battery Interfaces. Nano Lett. 2017, 17, 6974–6982. [Google Scholar] [CrossRef]
- Gao, B.; Jalem, R.; Ma, Y.; Tateyama, Y. Li+ Transport Mechanism at the Heterogeneous Cathode/Solid Electrolyte Interface in an All-Solid-State Battery via the First-Principles Structure Prediction Scheme. Chem. Mat. 2020, 32, 85–96. [Google Scholar] [CrossRef]
- Kammampata, S.P.; Thangadurai, V. Cruising in ceramics—Discovering new structures for all-solid-state batteries—Fundamentals, materials, and performances. Ionics 2018, 24, 639–660. [Google Scholar] [CrossRef]
- Thangadurai, V.; Chen, B. Solid Li- and Na-Ion Electrolytes for Next Generation Rechargeable Batteries. Chem. Mat. 2022, 34, 6637–6658. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics 2006, 12, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.W.; Wang, S.F. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Yao, P.H.; Yu, H.B.; Ding, Z.Y.; Liu, P.C.; Lu, J.; Lavorgna, M.; Wu, J.W.; Liu, X.J. Review on polymer-based composite electrolytes for lithium batteries. Front. Chem. 2019, 7, 00522. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond. Small 2022, 18, 2103617. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K.S. Review on composite polymer electrolytes for lithium batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef] [Green Version]
- Blake, A.J.; Kohlmeyer, R.R.; Hardin, J.O.; Carmona, E.A.; Maruyama, B.; Berrigan, J.D.; Huang, H.; Durstock, M.F. 3D Printable ceramic-polymer electrolytes for flexible high-performance Li-ion batteries with enhanced thermal stability. Adv. Energy Mater. 2017, 7, 1602920. [Google Scholar] [CrossRef]
- Mastragostino, M.; Soavi, F.; Zanelli, A. Improved composite materials for rechargeable lithium metal polymer batteries. J. Power Sources 1999, 81, 729–733. [Google Scholar] [CrossRef]
- Kumar, B.; Scanlon, L.G. Polymer-ceramic composite electrolytes: Conductivity and thermal history effects. Solid State Ion. 1999, 124, 239–254. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, R.L.; He, Y.B.; Li, F.Y.; Liu, M.; Li, B.H.; Yang, Q.H.; Cai, Q.; Kang, F.Y. SiO2 Hollow nanosphere-based composite solid electrolyte for lithium metal batteries to suppress lithium dendrite growth and enhance cycle life. Adv. Energy Mater. 2016, 6, 1502214. [Google Scholar] [CrossRef]
- Liu, W.; Lin, D.C.; Sun, J.; Zhou, G.M.; Cui, Y. Improved lithium ionic conductivity in composite polymer electrolytes with oxide-ion conducting nanowires. ACS Nano 2016, 10, 11407–11413. [Google Scholar] [CrossRef]
- Solarajan, A.K.; Murugadoss, V.; Angaiah, S. Dimensional stability and electrochemical behavior of ZrO2 incorporated electrospun PVDF-HFP based nanocomposite polymer membrane electrolyte for Li-ion capacitors. Sci. Rep. 2017, 7, 45390. [Google Scholar] [CrossRef] [Green Version]
- Sheng, O.W.; Jin, C.B.; Luo, J.M.; Yuan, H.D.; Huang, H.; Gan, Y.P.; Zhang, J.; Xia, Y.; Liang, C.; Zhang, W.K.; et al. Mg2B2O5 Nanowire enabled multifunctional solid-state electrolytes with high ionic conductivity, excellent mechanical properties, and flame-retardant performance. Nano Lett. 2018, 18, 3104–3112. [Google Scholar] [CrossRef]
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G. Ionic-conductivity and sinterability of lithium titanium phosphate system. Solid State Ion. 1990, 40–41, 38–42. [Google Scholar] [CrossRef]
- Kasper, H.M. A new series of rare Earth garnets LiN3+3M2Li+3O12(M = TE,W). Inorg. Chem. 1969, 8, 1000–1002. [Google Scholar] [CrossRef]
- Inaguma, Y.; Chen, L.Q.; Itoh, M.; Nakamura, T.; Uchida, T.; Ikuta, H.; Wakihara, M. High ionic-conductivity in lithium lanthanum titanate. Solid State Commun. 1993, 86, 689–693. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Sahami, S.; Shea, S.W.; Zhang, Z.M. Preparation and conductivity measurements of SiS2-Li2S glasses doped with LiBr and LiCl. Solid State Ion. 1986, 18–19, 368–371. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Gruzalski, G.R.; Zuhr, R.A.; Choudhury, A.; Luck, C.F.; Robertson, J.D. Fabrication and characterization of amorphous lithium electrolyte thin-films and rechargeable thin-film batteries. J. Power Sources 1993, 43, 103–110. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, E.B.; Lee, J.P.; Lee, S.W. All-Solid-State Batteries Comprising Composite Electrode. US Patent US2022231326 (A1), 21 July 2022. [Google Scholar]
- Ryu, J.H.; Kim, E.B.; Lee, J.P.; Lee, S.W. Method of Manufacturing Negative Electrode for All-Solid-State Batteries. US Patent US2022223830 (A1), 14 July 2022. [Google Scholar]
- Jung, H.R.; Choi, L.Y.; Han, H.E. Method of Manufacturing Positive Electrode for All-Solid-State Batteries and Positive Electrode for All-Solid-State Batteries Manufactured Using the Same. US Patent US2022190381 (A1), 16 June 2022. [Google Scholar]
- Jung, H.R.; Han, H.E.; Hah, H.J. Method of Manufacturing Positive Electrode Mixture for All-Solid-State Batteries and Positive Electrode Mixture for All-Solid-State Batteries Manufactured Using the Same. US Patent US2022181605 (A1), 9 June 2022. [Google Scholar]
- Takara, Y.; Takano, R. Solid Electrolyte Ceramic and Solid-State Battery. Patent WO2022107801 (A1), 27 May 2022. [Google Scholar]
- Takano, R. Solid Electrolyte Ceramic and Solid-State Battery. Patent WO2022107826 (A1), 27 May 2022. [Google Scholar]
- Takara, Y.; Takano, R. Solid Electrolyte Ceramic and Solid-State Battery. Patent WO2022107824 (A1), 27 May 2022. [Google Scholar]
- Terai, K.; Kondo, K.; Makino, T.; Sato, A.; Higuchi, H. Sulfide Solid Electrolyte Glass Ceramic and Manufacturing Method for Same. Patent WO2022075471 (A1), 14 April 2022. [Google Scholar]
- Fang, Z.; Fu, B.; Xu, Z.; Wu, M. Modified Sodium Ion Solid Electrolyte Ceramic Material with NASICON Type Structure as well as Preparation Method and Application of Modified Sodium Ion Solid Electrolyte Ceramic Material. Patent CN114349494 (A), 15 April 2022. [Google Scholar]
- Armand, M. The history of polymer electrolytes. Solid State Ion. 1994, 69, 309–319. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Ha, A.R.; Seong, J.Y.; Kim, Y.-G.; Song, I.W.; Min, H.-S.; Yoon, Y.S.; Kim, Y.-S.; Noh, S.W.; Jang, Y.J.; Lee, S.H.; et al. Cathode Active Material Comprising Ternary Oxide-Containing Coating for all-Solid-State Batteries and Method of Manufacturing Same. Patent CN114122343 (A), 1 March 2022. [Google Scholar]
- Ha, Y.K.; Choo, K.C.; Lim, D.H.; Oh, B.H.; Im, H.M. Composite Polymer Solid Electrolyte and Manufacturing Method Thereof. Patent KR20220023731 (A), 2 March 2022. [Google Scholar]
- Lyu, P.; Zou, X.; Zhang, D.; Xie, S.; Chen, X.; He, Y. Preparation Method of Polymer Solid Electrolyte Based on Lithium Metal Battery. Patent CN114221038 (A), 22 March 2022. [Google Scholar]
- Shang, D.; Wang, Y. Polymer Solid Electrolyte and Preparation Method and Application Thereof. Patent CN114171789 (A), 11 March 2022. [Google Scholar]
- Yang, C.C.; Wu, Y.X.; Kumlachew, Z.W. Method for Fabrication of Hybrid Solid Electrolyte Membrane, and All-Solid-State Lithium Battery including the Hybrid Solid Electrolyte Membrane. Patent JP2022029430 (A), 17 February 2022. [Google Scholar]
- Ha, Y.K.; Choo, K.C.; Lim, D.H. Hybrid Solid Electrolyte Separator Using Inorganic Fiber and Secondary Battery Using the Same. Patent KR20210146201 (A), 3 December 2021. [Google Scholar]
- Kim, H.S.; Kim, M.Y.; Jeon, S.H.; Ban, H.J.; Lim, J.; Lee, J.H. All Solid Lithium Secondary Battery Having High Voltage Stability Comprising Sulfide Based and Oxide-Based Hybrid Solid Electrolyte and Method of Preparing Same. Patent KR20210058101 (A), 24 May 2021. [Google Scholar]
- Fraunhofer ISI. Solid-State Battery Roadmap 2035+; Fraunhofer ISI: Karlsruhe, Germany, 2022. [Google Scholar]














| Battery Chemistry | Cell Potential/V | Theoretical Specific Energy/Wh kg–1 |
|---|---|---|
| Li-S2Li + S = Li2S | 2.2 | 2567 |
| Li-air (non-aqueous) 2Li + O2 = Li2O2 | 3.0 | 3505 |
| Li-air (aqueous) 2Li + ½O2 + H2O = 2LiOH | 3.2 | 3582 |
| Contemporary Li-ion 0.5C6Li + Li0.5CoO2 = 3C + LiCoO2 | 3.8 | 387 |
| Material | Amount of Graphene | Performance |
|---|---|---|
| Li4Ti5/holey-graphene | 50 wt.% | 98 mAh cm3 at 17.5 A g−1; 84% capacity retention after 1000 cycles at 7 A g−1 |
| Li4Ti5/graphene | 5 wt.% | 122 mAh cm3 at 30°C; 124.5 mA g−1; 98% capacity retention after 300 cycles at 20°C |
| Graphene-MnO2-GNRs | 68 wt.% | 300 mAh cm3 at 612 mAg−1 after 250 cycles at 0.4 A g−1 |
| MoS2-graphene | 4.7 wt.% | 570 mAh cm3 at 1A g−1; 894.1 mAh g−1 after 100 cycles at 0.1 A g−1 |
| Graphene anchored with Co3O4 | 24.6 wt.% | 484 mAh g−1 at 0.5A g−1; 935 mAh g−1 after 30 cycles at 0.1 A g−1 and a specific current of 0.05 Ag−1 |
| Solid-State Electrolyte | Composition | Ionic Conductivity S cm−1 |
|---|---|---|
| NASICON-like | Li1.4Al0.4Ti1.6[PO4]3 | 1.12·10−3 |
| LISICON-like | Li3.25Ge0.25P0.75S4 | 10−2 |
| thio-LISICON | Li9.54Si1.74P1.74S11.7Cl0.3 | 1.25·10−1 |
| Li3.25Ge0.25P0.75S4 | 10−2·10−3 | |
| Li9.54Si1.74P1.44S11.7Cl0.3 | 2.5·10−2 | |
| Anti-perovskites | Li3OX (X = Cl or Cl or Br) | >10−3 |
| Garnets | Li6.55La2.5BaZrTaO12 | 6·10−3 |
| Li6.5La3Zr1.5Ta0.5O12 | 0.75·10−3 | |
| Li6.25La3Zr2Al0.25O12 | 0.68·10−3 | |
| Li6.25La3Zr2Ta0.25Ga0.2O12 | 1.04·10−3 | |
| Li2S-SiS2 based | 95[0.6Li2S0.4SiS2]. 5Li4SiO4 | 10−3 |
| Li2S-P2S5 based | 70Li2S-30P2S5 glass ceramic | 3.2·10−3 |
| Activity | Devices |
|---|---|
| EVs, HEVs, EVs with two-wheel battery swapping functionality, electric bicycles, hybrid vehicles | |
| Consumer electronics | Autonomous sensor devices, smart homes (HVAC, security systems, lights); automotive (infotainment systems, sensors); logistics (asset tracking); wearables dedicated to the needs of next generation IoT edge nodes; miniature devices; electronics; standby power supplies; portable devices, the Internet of Things; emergency power protection; watches; autonomous sensors; Real Time Clock (RTC); products with semiconductors; automotive electrical equipment; safety UPS systems |
| Medicine/Health | Medical devices (biometric monitoring); medical implants; health and fitness applications; other medical applications |
| Aerospace | Satellites |
| Industrial | Factory/infrastructure/industrial equipment; rural electrification; hybrid power units for industries manufacturing, and production; 3C consumption industries; patrol inspection security systems; aerial photography and related industries; robotics and AI; IoT devices |
| Stationary energy storage | Integration of renewables; energy & utilities; localized power sources; power bridging; grid storage; large-scale energy storage |
| Military | Aviation; marine; defense |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bubulinca, C.; Kazantseva, N.E.; Pechancova, V.; Joseph, N.; Fei, H.; Venher, M.; Ivanichenko, A.; Saha, P. Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use. Batteries 2023, 9, 157. https://doi.org/10.3390/batteries9030157
Bubulinca C, Kazantseva NE, Pechancova V, Joseph N, Fei H, Venher M, Ivanichenko A, Saha P. Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use. Batteries. 2023; 9(3):157. https://doi.org/10.3390/batteries9030157
Chicago/Turabian StyleBubulinca, Constantin, Natalia E. Kazantseva, Viera Pechancova, Nikhitha Joseph, Haojie Fei, Mariana Venher, Anna Ivanichenko, and Petr Saha. 2023. "Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use" Batteries 9, no. 3: 157. https://doi.org/10.3390/batteries9030157