High-Entropy Metal Oxide (NiMnCrCoFe)3O4 Anode Materials with Controlled Morphology for High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis of (FeCoNiCrMn)3O4 HEMO
2.2. Battery Fabrication
2.3. Material Characterization and Electrochemical Measurements
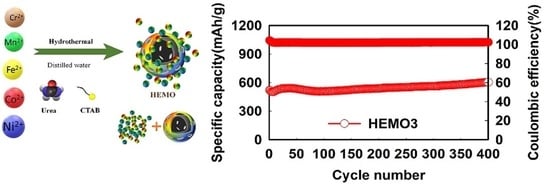
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, J.; Zhao, D.; Zhang, C. An overview of electricity powered vehicles: Lithium-ion battery energy storage density and energy conversion efficiency. Renew. Energy 2020, 162, 1629–1648. [Google Scholar] [CrossRef]
- Zheng, Q.; Watanabe, M.; Iwatate, Y.; Azuma, D.; Shibazaki, K.; Hiraga, Y.; Kishita, A.; Nakayasu, Y. Hydrothermal leaching of ternary and binary lithium-ion battery cathode materials with citric acid and the kinetic study. J. Supercrit. Fluids 2020, 165, 104990. [Google Scholar] [CrossRef]
- Fang, F.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.M. The regulating role of carbon nanotubes and graphene in lithium-ion and lithium-sulfur batteries. Adv. Mater. 2019, 31, 1800863. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, L.B.; Wei, H.X.; Zhang, X.H.; He, Z.J.; Li, Y.J.; Zheng, J.C. Enhancement on structural stability of Ni-rich cathode materials by in-situ fabricating dual-modified layer for lithium-ion batteries. Nano Energy 2019, 65, 104043. [Google Scholar] [CrossRef]
- Liang, C.; Gao, M.; Pan, H.; Liu, Y.; Yan, M. Lithium alloys and metal oxides as high-capacity anode materials for lithium-ion batteries. J. Alloys Compd. 2013, 575, 246–256. [Google Scholar] [CrossRef]
- Yao, H.L.; Gao, S.S.; Fu, Z.Q.; Bao, W.C.; Cui, Z.H.; Li, Y.Q.; Xu, F.F. Encapsulating manganese oxide nanoparticles within conducting polypyrrole via in situ redox reaction and oxidative polymerization for long-life lithium-ion batteries. Rare Met. 2021, 40, 2415–2423. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.L.; Jin, E.M.; Moon, S.G.; Jeong, S.M. Optimization of B2O3 coating process for NCA cathodes to achieve long-term stability for application in lithium ion batteries. Energy 2021, 222, 119913. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and recent progress on silicon-based anode materials for next-generation lithium-ion batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Baboukani, A.; Khakpour, I.; Adelowo, E.; Drozd, V.; Shang, W.; Wang, C. High-performance red phospho-rus-sulfurized polyacrylonitrile composite by electrostatic spray deposition for lithium-ion batteries. Electrochim. Acta 2020, 345, 136227. [Google Scholar] [CrossRef]
- Li, X.; Dhanabalan, A.; Wang, C. Enhanced electrochemical performance of porous NiO–Ni nanocomposite anode for lithium ion batteries. J. Power Sources 2011, 5, 9625–9630. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, D.; Zhang, X. Porous NiO materials prepared by solid-state thermolysis of a Ni-MOF crystal for lithium-ion battery anode. Nano Struct. Nano Objects 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, H.; Bu, Y.; Zhang, H.; Zhao, B.; Yang, C. Porous Co3O4 nanofibers surface-modified by reduced graphene oxide as a durable, high-rate anode for lithium ion battery. Electrochim. Acta 2017, 228, 241–250. [Google Scholar] [CrossRef]
- Huang, G.; Xu, S.; Lu, S.; Li, L.; Sun, H. Porous polyhedral and fusiform Co3O4 anode materials for high-performance lithium-ion batteries. Electrochim. Acta 2014, 135, 420–427. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Yang, M.; Lv, F.; Cao, L.; Tang, Y.; Sun, R.; Lu, Z. Large-scale fabrication of porous carbon-decorated iron oxide microcuboids from Fe–MOF as high-performance anode materials for lithium-ion batteries. RSC Adv. 2015, 5, 7356–7362. [Google Scholar] [CrossRef]
- Yu, A.; Park, H.W.; Davies, A.; Higgins, D.C.; Chen, Z.; Xiao, X. Free-standing layer-by-layer hybrid thin film of graphene-MnO2 nanotube as anode for lithium ion batteries. J. Phys. Chem. Lett. 2011, 2, 1855–1860. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, X.; Shan, H.; Chen, C.; Yan, B.; Xiong, D.; Li, D. Enhanced anode performance of flower-like NiO/RGO nanocomposites for lithium-ion batteries. Mater. Chem. Phys. 2018, 217, 547–552. [Google Scholar] [CrossRef]
- Guo, R.; Huang, X.; Lin, Y.; Cao, Y. NiO/Carbon aerogel microspheres with plum-pudding structure as anode materials for lithium ion batteries. Materials 2020, 13, 2363. [Google Scholar] [CrossRef]
- Xiao, M.J.; Ma, B.; Zhang, H.; Li, X.Y.; Wang, Q.; Peng, Y.; Zhang, H.L. Hollow NiO/carbon pompons for efficient lithium ion storage. J. Mater. Chem. A 2022, 10, 21492–21502. [Google Scholar] [CrossRef]
- Dong, X.L.; Zhang, Y.T.; Cui, C.S.; Zeng, S.Y.; Fu, C.G.; Wang, L. Study on the binary transition metal oxide Mn2V2O7 structures for high performance lithium-ion batteries. J. Alloys Compd. 2022, 907, 164518. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, S.; Zhao, Y.; Kuang, Q.; Fan, Q.; Dong, Y. Binary metal oxide anode material, VOMoO4/C, with a high capacity and ultralong cycle-life for lithium ion batteries and its multi-electron reaction mechanism. Solid State Ion. 2020, 348, 115280. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Kim, H.; Seo, J.G. Constructive designing of ternary metal oxide as an anode material for high performance lithium-ion batteries. Int. J. Energy Res. 2021, 45, 16592–16602. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [Green Version]
- Qiu, N.; Chen, H.; Yang, Z.; Sun, S.; Wang, Y.; Cui, Y. A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O) with superior lithium storage performance. J. Alloys Compd. 2019, 777, 767–774. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Duan, C.; Mao, J.; Dong, Y.; Dong, K.; Wang, Z.; Luo, S.; Liu, Y.; Qi, X. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J. Alloys Compd. 2020, 844, 156158. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, N.; Wu, B.; Yang, Z.; Sun, S.; Wang, Y. A new spinel high-entropy oxide (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 with fast reaction kinetics and excellent stability as an anode material for lithium ion batteries. RSC Adv. 2020, 10, 9736–9744. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Li, N.; Su, Y.; Shou, H.; Bao, L.; Yang, W.; Zhang, L.; An, R.; Chen, S. Spinel/layered heterostructured cathode material for high-capacity and high-rate Li-ion batteries. Adv. Mater. 2013, 25, 3722–3726. [Google Scholar] [CrossRef]
- Pan, X.; Duan, X.; Lin, X.; Zong, F.; Tong, X.; Li, Q.; Wang, T. Rapid synthesis of Cr-doped γ-Fe2O3/reduced graphene oxide nanocomposites as high performance anode materials for lithium ion batteries. J. Alloys Compd. 2018, 732, 270–279. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef]
- Duan, C.; Tian, K.; Li, X.; Wang, D.; Sun, H.; Zheng, R.; Wang, Z.; Liu, Y. New spinel high-entropy oxides (FeCoNiCrMnXLi)3O4 (X = Cu, Mg, Zn) as the anode material for lithium-ion batteries. Ceram. Int. 2021, 47, 32025–32032. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Fang, C.; Meng, Y.S. Urea-based hydrothermal synthesis of LiNi0.5Co0.2Mn0.3O2 cathode material for Li-ion battery. J. Power Sources 2018, 394, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Sha, O.; Wang, S.; Qiao, Z.; Yuan, W.; Tang, Z.; Xu, Q.; Su, Y. Synthesis of spinel LiNi0.5Mn1.5O4 cathode material with excellent cycle stability using urea-based sol–gel method. Mater. Lett. 2012, 89, 251–253. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, M.; Wang, L.; Li, J. CTAB-assisted synthesis of ZnCo2O4 nanoparticles embedded in N-doped carbon as superior anode materials for lithium-ion battery. J. Alloys Compd. 2019, 780, 897–906. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Hussein, M.Z.; Taufiq-Yap, Y.H. The effect of sodium dodecyl sulfate (SDS) and cetyltrimethylammonium bromide (CTAB) on the Properties of ZnO synthesized by hydrothermal method. Int. J. Mol. Sci. 2012, 13, 13275–13293. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.W.; Shearing, P.R.; Brandon, N.P.; Harris, S.J.; García, R.E. Particle Size Polydispersity in Li-Ion Batteries. J. Electrochem. Soc. 2014, 161, A422–A430. [Google Scholar] [CrossRef] [Green Version]
- Lokcu, E.; Toparli, C.; Anik, M. Electrochemical Performance of (MgCoNiZn)1−xLixO High-Entropy Oxides in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 23860–23866. [Google Scholar] [CrossRef]
- Jin, E.M.; Kim, M.S.; Kim, T.K.; Shin, B.J.; Moon, J.H.; Jeong, S.M. Upcycling of silicon scrap collected from photovoltaic cell manufacturing process for lithium-ion batteries via transferred arc thermal plasma. Energy 2023, 262, 125447. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; Biasi, L.D.; Kubel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.L.; Chen, J.; Jin, B.; Jiang, Q.; Jin, E.M.; Jeong, S.M. Electrochemical performance of electrospun lotus–root–structure porous multichannel carbon nanotubes for lithium–sulfur battery applications. J. Electroanal. Chem. 2020, 878, 114564. [Google Scholar] [CrossRef]
- Yan, T.; Zhong, S.; Zhou, M.; Guo, X.; Hu, J.; Wang, F.; Zeng, F.; Zuo, S. High-efficiency method for recycling lithium from spent LiFePO4 cathode. Nanotechnol. Rev. 2020, 9, 1586–1593. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Patra, J.; Chang, J.K.; Ting, J.M. High entropy spinel oxide nanoparticles for superior lithiation–delithiation performance. J. Mater. Chem. A 2020, 8, 18963–18973. [Google Scholar] [CrossRef]
- Yang, L.; Xia, Y.; Qin, L.; Yuan, G.; Qiu, B.; Shi, J.; Liu, Z. Concentration-gradient LiMn0.8Fe0.2PO4 cathode material for high performance lithium ion battery. J. Power Sources 2016, 304, 293–300. [Google Scholar] [CrossRef]
- Duan, C.; Li, X.; Wang, D.; Wang, Z.; Sun, H.; Zheng, R.; Liu, Y. Nanosized high entropy spinel oxide (FeCoNiCrMn)3O4 as a highly active and ultra-stable electrocatalyst for the oxygen evolution reaction. Sustain. Energy Fuels 2022, 6, 1479–1488. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Song, Y.; Liu, K.; Huang, T.; Wang, X.; Zhang, C.; Li, J. Low-Temperature Synthesis of a Porous High-Entropy Transition-Metal Oxide as an Anode for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 26873–26881. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Z.; Mao, M.; Wang, H.; Li, Y.; Ma, J. Metal oxide/graphene composite anode materials for sodium-ion batteries. Energy Storage Mater. 2019, 16, 434–454. [Google Scholar] [CrossRef]
- Somo, T.R.; Mabokela, T.E.; Teffu, D.M.; Sekgobela, T.K.; Ramogayana, B.; Hato, M.J.; Modibane, K.D. A Comparative Review of Metal Oxide Surface Coatings on Three Families of Cathode Materials for Lithium Ion Batteries. Coatings 2021, 11, 744. [Google Scholar] [CrossRef]
- Shobana, M.K. Metal oxide coated cathode materials for Li ion batteries—A review. J. Alloys Compd. 2019, 802, 477–487. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Banis, M.N.; Lushington, A.; Li, R.; Cai, M.; Sun, X. Atomic layer deposition of solid-state electrolyte coated cathode materials with superior high-voltage cycling behavior for lithium ion battery application. Energy Environ. Sci. 2014, 7, 768–778. [Google Scholar] [CrossRef]
- Ai, G.; Hu, Q.; Zhang, L.; Dai, K.; Wang, J.; Xu, Z.; Huang, Y.; Zhang, B.; Li, D.; Zhang, T.; et al. Investigation of the Nanocrystal CoS2 Embedded in 3D Honeycomb-like Graphitic Carbon with a Synergistic Effect for High-Performance Lithium Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 33987–33999. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Lai, M.O.; Lu, L. Influence of Ru substitution on Li-rich 0.55Li2MnO3·0.45LiNi1/3Co1/3Mn1/3O2 cathode for Li-ion batteries. Electrochim. Acta 2012, 80, 187–195. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Zhang, K.; Yu, X.; Yuan, J.; Zhu, D.M.; Ozawa, K.; Qin, L.C. Effect of porous structural properties on lithiumion and sodium-ion storage: Illustrated by the example of a micro-mesoporous graphene1-x(MoS2)x anode. RSC Adv. 2021, 11, 34152. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Ryu, K.-S. Study of the lithium diffusion properties and high rate performance of TiNb6O17 as an anode in lithium secondary battery. Sci. Rep. 2017, 7, 16617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheradmandfard, M.; Minouei, H.; Tsvetkov, N.; Vayghan, A.K.; Kashani-Bozorg, S.F.; Kim, G.; Hong, S.I.; Kim, D.E. Ultrafast green microwave-assisted synthesis of high-entropy oxide nanoparticles for Li-ion battery applications. Mater. Chem. Phys. 2021, 262, 124265. [Google Scholar] [CrossRef]
- Xiang, H.Z.; Xie, H.X.; Chen, Y.X.; Zhang, H.; Mao, A.; Zheng, C.H. Porous spinel-type (Al0.2CoCrFeMnNi)0.58O4-δ high-entropy oxide as a novel high-performance anode material for lithium-ion batteries. J. Mater. Sci. 2021, 56, 8127–8142. [Google Scholar] [CrossRef]
- Tian, K.H.; Duan, C.Q.; Ma, Q.; Li, X.L.; Wang, Z.Y.; Sun, H.Y.; Luo, S.H.; Wang, D.; Liu, Y.G. High-entropy chemistry stabilizing spinel oxide (CoNiZnXMnLi)3O4 (X = Fe, Cr) for high-performance anode of Li-ion batteries. Rare Met. 2022, 41, 1265–1275. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, D.; Zhu, Q.; Sun, N.; Fu, F.; Xu, B. Plate-to-Layer Bi2MoO6/MXene-Heterostructured Anode for Lithium-Ion Batteries. Nano Micro Lett. 2019, 11, 81. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Huang, S.; Hu, J.; Fan, S.; Shang, Y.; Pam, M.E.; Li, X.; Wang, Y.; Xu, T.; Shi, Y.; et al. Boosting Sodium Storage of Fe1−xS/MoS2 Composite via Heterointerface Engineering. Nano Micro Lett. 2019, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Lv, H.; Chen, S.; Kopold, P.; van Aken, P.A.; Wu, X.; Maier, J.; Yu, Y. Peapod-like Li3VO4/N-Doped Carbon Nanowires with Pseudocapacitive Properties as Advanced Materials for High-Energy Lithium-Ion Capacitors. Adv. Mater. 2017, 29, 1700142. [Google Scholar] [CrossRef]
- Xiong, J.; Pan, Q.; Zheng, F.; Xiong, X.; Yang, C.; Hu, D.; Huang, C. N/S Co-doped Carbon Derived From Cotton as High Performance Anode Materials for Lithium Ion Batteries. Front. Chem. 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, Q.; Li, Q.; An, Q.; Zhao, Y.; Peng, Z.; Jiang, Y.; Tan, S.; Yan, M.; Mai, L. Pseudocapacitive layered iron vanadate nanosheets cathode for ultrahigh-rate lithium ion storage. Nano Energy 2018, 47, 294–300. [Google Scholar] [CrossRef]
- Sun, X.G.; Liao, C.; Baggetto, L.; Guo, B.; Unocic, R.R.; Veith, G.M.; Dai, S. Bis(fluoromalonato)borate (BFMB) anion based ionic liquid as an additive for lithium-ion battery electrolytes. J. Mater. Chem. A 2014, 2, 7606–7614. [Google Scholar] [CrossRef]
- Delaporte, N.; Wang, Y.; Zaghib, K. Pre-treatments of Lithium Foil Surface for Improving the Cycling Life of Li Metal Batteries. Front. Mater. 2019, 6, 267. [Google Scholar] [CrossRef]
- Dai, H.; Gu, X.; Dong, J.; Wang, C.; Lai, C.; Sun, S. Stabilizing lithium metal anode by octaphenyl polyoxyethylene-lithium complexation. Nat. Commun. 2020, 11, 643. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Gong, J.; Deng, Z.; Lang, Y.; Zong, B.; Guo, J.; Wang, L. Synthesis and electrochemical performances of LiNi0.5Mn1.5O4 spinels with different surface orientations for lithium-ion batteries. Ionics 2020, 26, 2187–2200. [Google Scholar] [CrossRef]










| 2-Theta (o) | (hkl) | d-Spacing (Å) | |
|---|---|---|---|
| Experimental Values from XRD | Calculated from HR-TEM | ||
| 18.4 | (111) | 4.83 | 4.85 |
| 30.2 | (220) | 2.89 | 2.89 |
| 35.6 | (311) | 2.52 | 2.48 |
| 43.2 | (400) | 2.09 | 2.08 |
| 57.2 | (511) | 1.61 | 1.62 |
| 62.8 | (440) | 1.48 | 1.48 |
| 75.3 | (622) | 1.26 | 1.26 |
| Electrodes | 10th Cycle | |||
|---|---|---|---|---|
| Rs | RSEI | Rct | DLi + | |
| HEMO1 | 3.2 | 4.8 | 15.8 | 6.852 × 10−15 |
| HEMO2 | 3.7 | 5.8 | 22.4 | 2.016 × 10−14 |
| HEMO3 | 3.6 | 3.7 | 13.3 | 3.734 × 10−14 |
| HEMO Components (Method) | Electrochemical Measurement | Refs. | |||
|---|---|---|---|---|---|
| Discharge Capacity (C-rate) (mAh g−1) | Cycle Retention | ||||
| 0.1 A g−1 | 2 A g−1 | 5 A g−1 | |||
| Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O (Solid-state) | 955 | 600 | -- | ~99% (2nd–300th/0.1 A g−1) | [25] |
| (FeCoNiCrMn)3O4 (Solid-state) | 586 | 269 | ~61% (2nd–300th/0.5 A g−1) | [26] | |
| (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 (Hydrothermal) | 571 | 268 | 96.2% (800th/2 A g−1) | [27] | |
| (FeCoNiCrMnZnLi)3O4 (Solid-state) | 577 | 173 | ~75% (100th/0.5 A g−1) | [31] | |
| (MgCoNiZn)0.65Li0.35O (Solid-state) | 925 | -- | ~90% (30th–140th/1 A g−1) | [37] | |
| (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O (Nebulized Spray Pyrolysis) | 525 | -- | ~94% (50th/0.2 A g−1) | [40] | |
| (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O (Hydrothermal) | 1072 | 500 | ~90% (200th/0.5 A g−1) | [43] | |
| (Mg, Cu, Ni, Co, Zn)O (Green microwave-assisted method) | 686 | 285 | 250 | ~99% (1000th/1 A g−1) | [55] |
| (Al0.2CoCrFeMnNi)0.58O4-δ (Solution combustion synthesis) | 1500 | 781 | ~40% (500th/0.2 A g−1) | [56] | |
| (CoNiZnFeMnLi)3O4 (conventional solid-phase method) | 665 | 225 | ~84% (100th/0.1 A g−1) | [57] | |
| ((NiMnCrCoFe)3O4 (Hydrothermal) | 1189 | 752 | 527 | ~100% (300th/5 A g−1) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.L.; Jin, E.M.; Sahoo, G.; Jeong, S.M. High-Entropy Metal Oxide (NiMnCrCoFe)3O4 Anode Materials with Controlled Morphology for High-Performance Lithium-Ion Batteries. Batteries 2023, 9, 147. https://doi.org/10.3390/batteries9030147
Wang XL, Jin EM, Sahoo G, Jeong SM. High-Entropy Metal Oxide (NiMnCrCoFe)3O4 Anode Materials with Controlled Morphology for High-Performance Lithium-Ion Batteries. Batteries. 2023; 9(3):147. https://doi.org/10.3390/batteries9030147
Chicago/Turabian StyleWang, Xuan Liang, En Mei Jin, Gopinath Sahoo, and Sang Mun Jeong. 2023. "High-Entropy Metal Oxide (NiMnCrCoFe)3O4 Anode Materials with Controlled Morphology for High-Performance Lithium-Ion Batteries" Batteries 9, no. 3: 147. https://doi.org/10.3390/batteries9030147