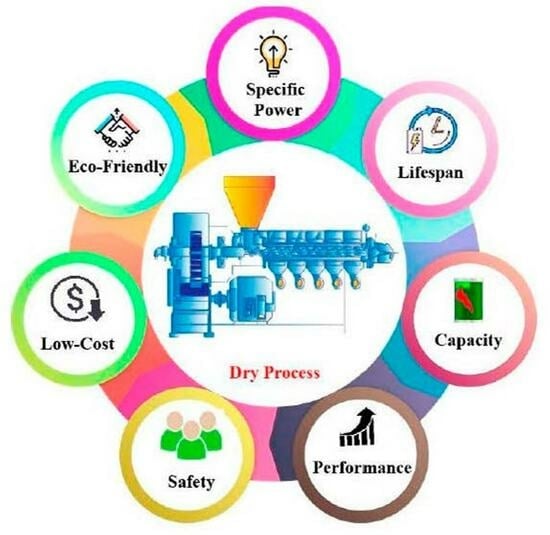
Engineering Dry Electrode Manufacturing for Sustainable Lithium-Ion Batteries
Abstract
:1. Introduction
1.1. Principal Parameters in Electrode Manufacturing for LIBs
- (i)
- Areal capacity
- (ii)
- Ionic tortuosity
- (iii)
- Production suitability
1.2. Disadvantages of the Wet Process
1.3. Progression Phases and Selective Approaches to Electrode Fabrication
2. Status of Lithium-Ion Batteries
2.1. Electrodes
2.1.1. Cathodes
2.1.2. Anodes
2.2. Electrolytes
2.3. Separator
2.4. Fabrication of Microporous Separator Materials by Using Dry and Wet Techniques
2.4.1. Wet Process
2.4.2. Dry Process
3. Dry Electrode Processing for LIBs
3.1. Electrodes
3.2. Dry Mixing and Coating
3.3. Dry Spraying Deposition
- Wet (lower loading): the electrode had a thickness of 32.6 µm, and it carried a mass loading value of 7.65 mg/cm2;
- Wet (higher loading): the electrode had a thickness of 52 µm, with a mass loading of 14.27 mg/cm2;
- Dry: the dry electrode had a thickness of 40.5 µm, with a mass loading of 10.07 mg/cm2.
- Wet (lower loading): the electrodes exhibited an initial discharge capacity of 156 mAh/g, with a capacity retention of 60% after 300 cycles when cycled between 3 V and 4.3 V at a rate of 0.5 C;
- Wet (higher loading): the electrode demonstrated an initial discharge capacity of 157 mAh/g, with a capacity retention of 65% after the same cycling conditions;
- Dry: the dry electrode displayed a primary discharge capability retention of 80% after the same cycling conditions.
- Wet (55 µm): the electrode showed a primary discharge of 145 mAh/g, with a capacity retention of 65% after 500 cycles;
- Dry (55 µm): the electrode exhibited an initial discharge capacity of 150 mAh/g, with capacity retention of over 80% after the same cycling conditions;
- Dry (55 µm): the electrode displayed a discharge capacity of 120 mAh/g at a high rate of 3 C;
- Dry (100 µm, 150 µm, and 200 µm): the electrode exhibited a discharge capacity of less than 20 mAh/g at the same high rate of 3 C.
- Low MW PVDF: the electrode displayed an initial discharge capacity of 160 mAh/g at a rate of 0.2 C, with capacity retention of 93% after 50 cycles at 0.5 C; however, at a higher rate of 5 C, the capacity retention dropped to 16.7%;
- High MW PVDF: the electrode exhibits a primary discharge capacity of 160 mAh/g at 0.2 C, with a capacity retention of 91% following 50 cycles.
3.4. Polymer Fibrillation
3.5. Extrusion and Melt Processing
4. Summary
- Binder selection: The choice of binder in the dry process is critical because it must provide sufficient cohesion to hold the active materials together without the aid of a solvent. Finding binders that can perform effectively in a dry environment is challenging, as they need to ensure structural integrity and maintain electrical contact between particles.
- Homogeneity of dry mixtures: Achieving a uniform mixture when combining materials of varying densities and particle sizes is more difficult in a dry process. The absence of a liquid medium can lead to segregation, making it challenging to obtain a consistent and homogenous mix, which is essential for the battery’s performance.
- Cohesion and adhesion: The dry process must ensure that the particles not only stick together (cohesion) but also adhere well to the current collectors (adhesion). Without the use of solvents, which often act as adhesives, maintaining the balance between cohesion and adhesion becomes a complex task.
- Equipment: Dry processing requires specialized equipment that can handle and mix particulate materials without causing damage or loss regarding material properties. This equipment must also be capable of pressing the mixture into a dense, uniform electrode film, which is a different technical challenge compared to the wet process.
- Compatibility with existing production lines: One of the significant hurdles is integrating the dry process with current production lines designed for wet processing. The infrastructure for wet processes is well established, and transitioning to dry methods may require substantial modifications or entirely new manufacturing lines, entailing significant investment and technical redesign.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| LIBs | Lithium-ion batteries |
| AM | Active materials |
| CA | Conductive additives |
| EVs | Electric vehicles |
| WP | Wet process |
| DP | Dry process |
| NMP | N-Methyl Pyrrolidone |
| SF | Solvent-free |
| ASSBs | All-solid-state batteries |
| NCA | Lithium Nickel Cobalt Aluminum Oxide |
| LNMO | Lithium Nickel Manganese Oxide |
| SiOx | Silicon Oxide |
| PE | Polyethylene |
| PP | Polypropylene |
| ESD | Electrostatic dry spraying deposition |
| NMC | Lithium-Nickel-Manganese-Cobalt Oxide |
| DSD | Dry spray deposition |
| LCO | Lithium-Cobalt-Oxide |
| MW | Molecular weight |
| PVDF | Polyvinylidene Fluoride |
| PTFE | Polytetrafluoroethylene |
| LFP | Lithium Iron Phosphate |
| LTO | Lithium titanate oxide |
| PPC | propylene carbonate |
| HNBR | Hydrogenated nitrile butadiene rubber |
| Tg | Glass transition temperature |
| Tm | Melting temperature |
| PW | Paraffin wax |
| SA | Stearic acid |
| LUMO | Lowest unoccupied molecular orbitals |
| CNTs | Carbon nanotubes |
References
- Toro, L.; Moscardini, E.; Baldassari, L.; Forte, F.; Falcone, I.; Coletta, J.; Toro, L. A Systematic Review of Battery Recycling Technologies: Advances, Challenges, and Future Prospects. Energies 2023, 16, 6571. [Google Scholar] [CrossRef]
- Zheng, M.; Salim, H.; Liu, T.; Stewart, R.A.; Lu, J.; Zhang, S. Intelligence-Assisted Predesign for the Sustainable Recycling of Lithium-Ion Batteries and Beyond. Energy Environ. Sci. 2021, 14, 5801–5815. [Google Scholar] [CrossRef]
- Shu, X.; Guo, Y.; Yang, W.; Wei, K.; Zhu, G. Life-cycle assessment of the environmental impact of the batteries used in pure electric passenger cars. Energy Rep. 2021, 7, 2302–2315. [Google Scholar] [CrossRef]
- Nagaura, T. Lithium ion rechargeable battery. Prog. Batteries Sol. Cells 1990, 9, 209. [Google Scholar]
- Broadhead, J.; Gibbard, H.F.; Kuo, H.; Chi, I.; Bowden, W. High-Energy, High-Power Lithium-Ion Rechargeable Cells. In Proceedings of the Symposium on Rechargeable Lithium and Lithium-Ion Batteries; The Electrochemical Society: Pennington, NJ, USA, 1995; Volume 94, p. 370. [Google Scholar]
- Ram, M.; Aghahosseini, A.; Breyer, C. Job creation during the global energy transition towards 100% renewable power system by 2050. Technol. Forecast. Soc. Change 2020, 151, 119682. [Google Scholar] [CrossRef]
- Degen, F.; Krätzig, O. Future in Battery Production: An Extensive Benchmarking of Novel Production Technologies as Guidance for Decision Making in Engineering. IEEE Trans. Eng. Manag. 2022, 71, 1038–1056. [Google Scholar] [CrossRef]
- Global EV Outlook 2023. Available online: https://iea.blob.core.windows.net/assets/dacf14d2-eabc-498a-8263-9f97fd5dc327/GEVO2023.pdf (accessed on 5 May 2023).
- World Economic Forum. A Vision for a Sustainable Battery Value Chain in 2030. Available online: https://www3.weforum.org/docs/WEF_A_Vision_for_a_Sustainable_Battery_Value_Chain_in_2030_Report.pdf (accessed on 9 May 2023).
- Degen, F. Lithium-Ion Battery Cell Production in Europe: Scenarios for Reducing Energy Consumption and Greenhouse Gas Emissions Until 2030. J. Ind. Ecol. 2023, 27, 964–976. [Google Scholar] [CrossRef]
- Musk, E. Tesla Battery Day 2020; Tesla, Inc.: Fremont, CA, USA, 2020; Available online: https://www.tesla.com/2020shareholdermeeting (accessed on 10 May 2023).
- Wang, M.; Dong, X.; Escobar, I.C.; Cheng, Y.T. Lithium Ion Battery Electrodes Made Using Dimethyl Sulfoxide (DMSO) A Green Solvent. ACS Sustain. Chem. Eng. 2020, 8, 11046–11051. [Google Scholar] [CrossRef]
- Haufroid, V.; Jaeger, V.K.; Jeggli, S.; Eisenegger, R.; Bernard, A.; Friedli, D.; Lison, D.; Hotz, P. Biological Monitoring and Health Effects of Low-Level Exposure to N-Methyl-2-Pyrrolidone: A Cross-Sectional Study. Int. Arch. Occup. Environ. Health 2014, 87, 663–674. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Esan, O.C.; An, L. Organic Electrolytes Recycling From Spent Lithium-Ion Batteries. Glob. Chall. 2022, 6, 2200050. [Google Scholar] [CrossRef]
- Ryu, M.; Hong, Y.K.; Lee, S.Y.; Park, J.H. Ultrahigh Loading Dry-Process for Solvent-Free Lithium-Ion Battery Electrode Fabrication. Nat. Commun. 2023, 14, 1316. [Google Scholar] [CrossRef]
- Chordia, M.; Nordelöf, A.; Ellingsen, L.A.W. Environmental Life Cycle Implications of Upscaling Lithium-Ion Battery Production. Int. J. Life Cycle Assess. 2021, 26, 2024–2039. [Google Scholar] [CrossRef]
- Fernandez-Diaz, L.; Castillo, J.; Sasieta-Barrutia, E.; Arnaiz, M.; Cabello, M.; Judez, X.; Villaverde, A. Mixing Methods for Solid State Electrodes: Techniques, Fundamentals, Recent Advances, and Perspectives. Chem. Eng. J. 2023, 464, 142469. [Google Scholar] [CrossRef]
- Valikangas, J.; Sliz, R.; Silva Santos, H.; Vilmi, P.; Rieppo, L.; Hu, T.; Fabritius, T. Suitable Cathode NMP Replacement for Efficient Sustainable Printed Li-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 4047–4058. [Google Scholar]
- Daniel, C.; Li, J.; Mohanty, D.; Wood, D.L., III. Thick Low-Cost, High-Power Lithium-Ion Electrodes via Aqueous Processing. In Proceedings of the US Department of Energy Hydrogen and Fuel Cells Program Annual Merit Review and Peer Evaluation Meeting, Washington, DC, USA, 6–10 June 2016; Office of Energy Efficiency & Renewable Energy: Washington, DC, USA, 2016. [Google Scholar]
- Lyckfeldt, O.; Orlenius, J.; Kasvayee, K.A.; Johander, P. Water Based Processing of LiFePO4/C Cathode Material for Li-Ion Batteries Utilizing Freeze Granulation. J. Power Sources 2012, 213, 119–127. [Google Scholar]
- Chen, C.F.; Stein, M., IV; Robles, D.J.; Rhodes, C.; Mukherjee, P.P. Non-Aqueous Electrode Processing and Construction of Lithium-Ion Coin Cells. JoVE (J. Vis. Exp.) 2016, 108, e53490. [Google Scholar]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-Ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef]
- Liu, J.; Ludwig, B.; Liu, Y.; Zheng, Z.; Wang, F.; Tang, M.; Wang, Y. Scalable Dry Printing Manufacturing to Enable Long-Life and High Energy Lithium-Ion Batteries. Adv. Mater. Technol. 2017, 2, 1700106. [Google Scholar] [CrossRef]
- Ludwig, B.; Liu, J.; Chen, I.M.; Liu, Y.; Shou, W.; Wang, Y.; Pan, H. Understanding Interfacial-Energy-Driven Dry Powder Mixing for Solvent-Free Additive Manufacturing of Li-Ion Battery Electrodes. Adv. Mater. Interfaces 2017, 4, 1700570. [Google Scholar] [CrossRef]
- Wood, D.L., III; Wood, M.; Li, J.; Du, Z.; Ruther, R.E.; Hays, K.A.; Belharouak, I. Perspectives on the Relationship Between Materials Chemistry and Roll-to-Roll Electrode Manufacturing for High-Energy Lithium-Ion Batteries. Energy Storage Mater. 2020, 29, 254–265. [Google Scholar] [CrossRef]
- Kato, Y.; Shiotani, S.; Morita, K.; Suzuki, K.; Hirayama, M.; Kanno, R. All-Solid-State Batteries with Thick Electrode Configurations. J. Phys. Chem. Lett. 2018, 9, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Duong, H. Electrochemical Performance of Dry Battery Electrode. ECS Meet. Abstr. 2018, 233, 365. [Google Scholar] [CrossRef]
- Duong, H.; Shin, J.; Yudi, Y. Dry Electrode Coating Technology. In Proceedings of the 48th Power Sources Conference, Denver, CO, USA, 11–14 June 2018; Volume 3, pp. 34–37. [Google Scholar]
- Schälicke, G.; Landwehr, I.; Dinter, A.; Pettinger, K.H.; Haselrieder, W.; Kwade, A. Solvent-Free Manufacturing of Electrodes for Lithium-Ion Batteries via Electrostatic Coating. Energy Technol. 2020, 8, 1900309. [Google Scholar] [CrossRef]
- Shin, J.; Yudi, Y.; Magsino, P.; Wong, W.; Duong, H. Dry Processed Nickel-Rich Layered Transition Metal Oxide Cathode Electrode. ECS Meet. Abstr. 2019, 235, 317. [Google Scholar] [CrossRef]
- Hippauf, F.; Schumm, B.; Doerfler, S.; Althues, H.; Fujiki, S.; Shiratsuchi, T.; Kaskel, S. Overcoming Binder Limitations of Sheet-Type Solid-State Cathodes Using a Solvent-Free Dry-Film Approach. Energy Storage Mater. 2019, 21, 390–398. [Google Scholar] [CrossRef]
- Sahore, R.; Wood, D.L., III; Kukay, A.; Grady, K.M.; Li, J.; Belharouak, I. Towards Understanding of Cracking During Drying of Thick Aqueous-Processed LiNi0.8Mn0.1Co0.1O2 Cathodes. ACS Sustain. Chem. Eng. 2020, 8, 3162–3169. [Google Scholar] [CrossRef]
- Lacey, S.D.; Walsh, E.D.; Hitz, E.; Dai, J.; Connell, J.W.; Hu, L.; Lin, Y. Highly Compressible, Binderless and Ultrathick Holey Graphene-Based Electrode Architectures. Nano Energy 2017, 31, 386–392. [Google Scholar] [CrossRef]
- Hawley, W.B.; Parejiya, A.; Bai, Y.; Meyer, H.M., III; Wood, D.L., III; Li, J. Lithium and Transition Metal Dissolution Due to Aqueous Processing in Lithium-Ion Battery Cathode Active Materials. J. Power Sources 2020, 466, 228315. [Google Scholar] [CrossRef]
- Wu, X.; Xia, S.; Huang, Y.; Hu, X.; Yuan, B.; Chen, S.; Liu, W. High-Performance, Low-Cost, and Dense-Structure Electrodes with High Mass Loading for Lithium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1903961. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, J.; Baumann, D.; Peng, L.; Xu, Y.; Shakir, I.; Huang, Y.; Duan, X. Hierarchical 3D Electrodes for Electrochemical Energy Storage. Nat. Rev. Mater. 2019, 4, 45–60. [Google Scholar] [CrossRef]
- Peng, H.J.; Huang, J.Q.; Cheng, X.B.; Zhang, Q. Review on High-Loading and High-Energy Lithium–Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, T.; Ai, X.; Liang, C. Aligning Academia and Industry for Unified Battery Performance Metrics. Nat. Commun. 2018, 9, 5262. [Google Scholar] [CrossRef] [PubMed]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-Lithium-Ion Battery Cell Production and Its Compatibility with Lithium-Ion Cell Production Infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Kim, H.M.; Yoo, B.I.; Yi, J.W.; Choi, M.J.; Yoo, J.K. Solvent-Free Fabrication of Thick Electrodes in Thermoplastic Binders for High Energy Density Lithium-Ion Batteries. Nanomaterials 2022, 12, 3320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chouchane, M.; Shojaee, S.A.; Winiarski, B.; Liu, Z.; Li, L.; Meng, Y.S. Coupling of Multiscale Imaging Analysis and Computational Modeling for Understanding Thick Cathode Degradation Mechanisms. Joule 2023, 7, 201–220. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for Lithium-Ion Battery Safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Li, B. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Li, H.; Peng, L.; Wu, D.; Wu, J.; Zhu, Y.J.; Hu, X. Ultrahigh-Capacity and Fire-Resistant LiFePO4-Based Composite Cathodes for Advanced Lithium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1802930. [Google Scholar] [CrossRef]
- Kaur, S.; Santra, S. Application of Guar Gum and its Derivatives as Green Binder/Separator for Advanced Lithium-Ion Batteries. ChemistryOpen 2022, 11, e202100209. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, J.H.; Seo, J.K.; Ran, W.T.A.; Whang, D.; Hwang, S.M.; Kim, Y.J. Graphene/PVDF Composites for Ni-Rich Oxide Cathodes Toward High-Energy Density Li-Ion Batteries. Materials 2021, 14, 2271. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Khau, B.V.; Davies, G.; Hertzberg, B.; Steingart, D.A.; Arias, A.C. A High Areal Capacity Flexible Lithium-Ion Battery with a Strain-Compliant Design. Adv. Energy Mater. 2015, 5, 1401389. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Vanpeene, V.; Heitz, A.; Herkendaal, N.; Soucy, P.; Douillard, T.; Roué, L. Transforming Silicon Slag into High-Capacity Anode Material for Lithium-Ion Batteries. Battery Energy 2022, 1, 20220016. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, H.; Liu, R.; Zhang, W.; Han, S.; Han, J.; Ma, Z.F. In-Situ Processing Nano-Porous Silicon into 3D Conductive Structure as High-Capacity Anode for Lithium-Ion Batteries. Sci. China Mater. 2023, 66, 493–504. [Google Scholar] [CrossRef]
- Li, J.; Fan, S.; Xiu, H.; Wu, H.; Huang, S.; Wang, S.; Xiong, C. TiO2-Coated Silicon Nanoparticle Core-Shell Structure for High-Capacity Lithium-Ion Battery Anode Materials. Nanomaterials 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wu, D.; Li, H.; Chen, L.; Wu, F. Long-Life High-Capacity Lithium Battery with Liquid Organic Cathode and Sulfide Solid Electrolyte. Battery Energy 2023, 2, 20220059. [Google Scholar] [CrossRef]
- Zuluaga-Gómez, C.C.; Plaza-Rivera, C.O.; Tripathi, B.; Katiyar, R.K.; Pradhan, D.K.; Morell, G.; Katiyar, R.S. Holey Graphene/Ferroelectric/Sulfur Composite Cathodes for High-Capacity Lithium–Sulfur Batteries. ACS Omega 2023, 8, 13097–13108. [Google Scholar] [CrossRef]
- Park, S.H.; King, P.J.; Tian, R.; Boland, C.S.; Coelho, J.; Zhang, C.; Nicolosi, V. High Areal-Capacity Battery Electrodes Enabled by Segregated Nanotube Networks. Nat. Energy 2019, 4, 560–567. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.M.; Cho, S.K.; Kim, N.Y.; Lee, S.Y. Redox-Homogeneous, Gel Electrolyte-Embedded High-Mass-Loading Cathodes for High-Energy Lithium Metal Batteries. Nat. Commun. 2022, 13, 2541. [Google Scholar] [CrossRef]
- Sawada, S.; Yoshida, H.; Luski, S.; Markevich, E.; Salitra, G.; Elias, Y.; Aurbach, D. Stable High-Capacity Elemental Sulfur Cathodes with Simple Process for Lithium Sulfur Batteries. Molecules 2023, 28, 4568. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, T.G.; Kim, H.; Park, J.W.; Hwang, H.J.; Hwang, H.; Jun, Y.S. Thick Free-Standing Electrode Based on Carbon–Carbon Nitride Microspheres with Large Mesopores for High-Energy-Density Lithium–Sulfur Batteries. Carbon Energy 2021, 3, 410–423. [Google Scholar] [CrossRef]
- McCloskey, B.D. Attainable Gravimetric and Volumetric Energy Density of Li–S and Li Ion Battery Cells with Solid Separator-Protected Li Metal Anodes. J. Phys. Chem. Lett. 2015, 6, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Demortière, A.; De Andrade, V.; Morcrette, M.; Seznec, V. Thick Binder-Free Electrodes for Li–Ion Battery Fabricated Using Templating Approach and Spark Plasma Sintering Reveals High Areal Capacity. Adv. Energy Mater. 2018, 8, 1703031. [Google Scholar] [CrossRef]
- Hu, L.; La Mantia, F.; Wu, H.; Xie, X.; McDonough, J.; Pasta, M.; Cui, Y. Lithium-Ion Textile Batteries with Large Areal Mass Loading. Adv. Energy Mater. 2011, 1, 1012–1017. [Google Scholar] [CrossRef]
- Wang, J.S.; Liu, P.; Sherman, E.; Verbrugge, M.; Tataria, H. Formulation and characterization of ultra-thick electrodes for high energy lithium-ion batteries employing tailored metal foams. J. Power Sources 2011, 196, 8714–8718. [Google Scholar] [CrossRef]
- Peled, E.; Patolsky, F.; Golodnitsky, D.; Freedman, K.; Davidi, G.; Schneier, D. Tissue-like silicon nanowires-based three-dimensional anodes for high-capacity lithium ion batteries. Nano Lett. 2015, 15, 3907–3916. [Google Scholar] [CrossRef]
- Higgins, T.M.; Park, S.H.; King, P.J.; Zhang, C.; McEvoy, N.; Berner, N.C.; Coleman, J.N. A commercial conducting polymer as both binder and conductive additive for silicon nanoparticle-based lithium-ion battery negative electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Xiao, Y.; Hwang, J.Y.; Belharouak, I.; Sun, Y.K. Novel strategy to improve the Li-storage performance of micro silicon anodes. J. Power Sources 2017, 348, 302–310. [Google Scholar] [CrossRef]
- Hong, H.; Salem, D.R.; Christensen, G.L.; Yang, R.; Younes, H.A. High Capacity Electrodes. U.S. Patent 11,626,584, 11 April 2023. [Google Scholar]
- Wang, M.J.; Kazyak, E.; Dasgupta, N.P.; Sakamoto, J. Transitioning solid-state batteries from lab to market: Linking electro-chemo-mechanics with practical considerations. Joule 2021, 5, 1371–1390. [Google Scholar] [CrossRef]
- Ye, G.; Zhao, M.; Hou, L.P.; Chen, W.J.; Zhang, X.Q.; Li, B.Q.; Huang, J.Q. Evaluation on a 400 Wh kg−1 lithium–sulfur pouch cell. J. Energy Chem. 2022, 66, 24–29. [Google Scholar] [CrossRef]
- Cerbelaud, M.; Lestriez, B.; Videcoq, A.; Ferrando, R.; Guyomard, D. Understanding the structure of electrodes in Li-ion batteries: A numerical study. J. Electrochem. Soc. 2015, 162, A1485. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, M.; Zhou, X.; Zhou, Z. Structural design for anodes of lithium-ion batteries: Emerging horizons from materials to electrodes. Mater. Horiz. 2015, 2, 553–566. [Google Scholar] [CrossRef]
- Shodiev, A.; Chouchane, M.; Gaberscek, M.; Arcelus, O.; Xu, J.; Oularbi, H.; Franco, A.A. Deconvoluting the benefits of porosity distribution in layered electrodes on the electrochemical performance of Li-ion batteries. Energy Storage Mater. 2022, 47, 462–471. [Google Scholar] [CrossRef]
- Santoki, J.; Daubner, S.; Schneider, D.; Kamlah, M.; Nestler, B. Effect of tortuosity, porosity, and particle size on phase-separation dynamics of ellipsoid-like particles of porous electrodes: Cahn–Hilliard-type phase-field simulations. Modell. Simul. Mater. Sci. Eng. 2021, 29, 06501. [Google Scholar] [CrossRef]
- Ragones, H.; Menkin, S.; Kamir, Y.; Gladkikh, A.; Mukra, T.; Kosa, G.; Golodnitsky, D. Towards smart free form-factor 3D printable batteries. Sustain. Energy Fuels 2018, 2, 1542–1549. [Google Scholar] [CrossRef]
- Tjaden, B.; Brett, D.J.; Shearing, P.R. Tortuosity in electrochemical devices: A review of calculation approaches. Int. Mater. Rev. 2018, 63, 47–67. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Demortière, A.; Fleutot, B.; Delobel, B.; Delacourt, C.; Cooper, S.J. The electrode tortuosity factor: Why the conventional tortuosity factor is not well suited for quantifying transport in porous Li-ion battery electrodes and what to use instead. NPJ Comput. Mater. 2020, 6, 123. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick electrode batteries: Principles, opportunities, and challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Kaiser, N.; Spannenberger, S.; Schmitt, M.; Cronau, M.; Kato, Y.; Roling, B. Ion transport limitations in all-solid-state lithium battery electrodes containing a sulfide-based electrolyte. J. Power Sources 2018, 396, 175–181. [Google Scholar] [CrossRef]
- Kehrwald, D.; Shearing, P.R.; Brandon, N.P.; Sinha, P.K.; Harris, S.J. Local tortuosity inhomogeneities in a lithium battery composite electrode. J. Electrochem. Soc. 2011, 158, A1393. [Google Scholar] [CrossRef]
- Vijayaraghavan, B.; Ely, D.R.; Chiang, Y.M.; García-García, R.; García, R.E. An analytical method to determine tortuosity in rechargeable battery electrodes. J. Electrochem. Soc. 2012, 159, A548. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Bazylak, A. Lattice Boltzmann Simulation of Tortuous Flow within Stochastic Porous Media and the Examination of the Tortuosity-Porosity Relationship. In Proceedings of the 224th ECS Meeting, San Francisco, CA, USA, 27 October–1 November 2013; Volume 224, p. 1228. [Google Scholar]
- Thorat, I.V.; Stephenson, D.E.; Zacharias, N.A.; Zaghib, K.; Harb, J.N.; Wheeler, D.R. Quantifying tortuosity in porous Li-ion battery materials. J. Power Sources 2009, 188, 592–600. [Google Scholar] [CrossRef]
- Röder, F.; Sonntag, S.; Schröder, D.; Krewer, U. Simulating the impact of particle size distribution on the performance of graphite electrodes in lithium-ion batteries. Energy Technol. 2016, 4, 1588–1597. [Google Scholar] [CrossRef]
- Lestariningsih, T.; Sabrina, Q.; Ratri, C.R.; Subhan, A.; Priyono, S. The Effect of LiBOB Addition on Solid Polymer Electrolyte (SPE) Production based PVDF-HFP/TiO2/LiTFSI on Ionic Conductivity for Lithium-Ion Battery Applications. J. Kimia Sains Apl. 2022, 25, 13–19. [Google Scholar] [CrossRef]
- Dyartanti, E.R.; Purwanto, A.; Widiasa, I.N.; Susanto, H. Ionic conductivity and cycling stability improvement of PVdF/nano-clay using PVP as polymer electrolyte membranes for LiFePO4 batteries. Membranes 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Lanceros-Méndez, S.; Costa, C.M. Electrode fabrication process and its influence in lithium-ion battery performance: State of the art and future trends. Electrochem. Commun. 2022, 135, 107210. [Google Scholar] [CrossRef]
- Yonaga, A.; Kawauchi, S.; Mori, Y.; Xuanchen, L.; Ishikawa, S.; Nunoshita, K.; Matsunaga, T. Effects of dry powder mixing on electrochemical performance of lithium-ion battery electrode using solvent-free dry forming process. J. Power Sources 2023, 581, 233466. [Google Scholar] [CrossRef]
- Erabhoina, H.; Thelakkat, M. Tuning of composition and morphology of LiFePO4 cathode for applications in all solid-state lithium metal batteries. Sci. Rep. 2022, 12, 5454. [Google Scholar] [CrossRef]
- Keppeler, M.; Tran, H.Y.; Braunwarth, W. The Role of Pilot Lines in Bridging the Gap Between Fundamental Research and Industrial Production for Lithium-Ion Battery Cells Relevant to Sustainable Electromobility: A Review. Energy Technol. 2021, 9, 2100132. [Google Scholar] [CrossRef]
- Duquesnoy, M.; Liu, C.; Dominguez, D.Z.; Kumar, V.; Ayerbe, E.; Franco, A.A. Machine learning-assisted multi-objective optimization of battery manufacturing from synthetic data generated by physics-based simulations. Energy Storage Mater. 2023, 56, 50–61. [Google Scholar] [CrossRef]
- Grant, P.S.; Greenwood, D.; Pardikar, K.; Smith, R.; Entwistle, T.; Middlemiss, L.A.; Cumming, D.J. Roadmap on Li-ion battery manufacturing research. J. Phys. Energy 2022, 4, 042006. [Google Scholar] [CrossRef]
- Chae, B.J.; Song, H.J.; Mun, J.; Yim, T. Effect of Sulfate-based Cathode-Electrolyte Interphases on Electrochemical Performance of Ni-rich Cathode Material. J. Electrochem. Sci. Technol. 2020, 11, 361–367. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.L.; Quass, J.D.; Li, J.; Ahmed, S.; Ventola, D.; Daniel, C. Technical and economic analysis of solvent-based lithium-ion electrode drying with water and NMP. Dry. Technol. 2018, 36, 234–244. [Google Scholar] [CrossRef]
- Hawley, W.B.; Meyer, H.M., III; Li, J. Enabling aqueous processing for LiNi0.80Co0.15Al0.05O2 (NCA)-based lithium-ion battery cathodes using polyacrylic acid. Electrochim. Acta 2021, 380, 138203. [Google Scholar] [CrossRef]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles–Critical issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- N-Methyl-2-Pyrrolidone NMP Solvents for Lithium Battery Applications. Available online: https://nanografi.com/battery-equipment/n-methyl-2-pyrrolidone-nmp-solvent-for-lithium-battery-cathode-materials-purity-99-90/ (accessed on 9 June 2023).
- Nelson, P.A.; Ahmed, S.; Gallagher, K.G.; Dees, D.W. Modeling the Performance and Cost of Lithium-Ion Batteries for Electric-Drive Vehicles; ANL/CSE-19/2; Argonne National Laboratory: Argonne, IL, USA, 2019. [Google Scholar]
- Emilsson, E.; Dahllöf, L. Lithium-Ion Vehicle Battery Production-Status 2019 on Energy Use, CO2 Emissions, Use of Metals, Products Environmental Footprint, and Recycling; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2019. [Google Scholar]
- Sitarek, K.; Stetkiewicz, J.; Wąsowicz, W. Evaluation of reproductive disorders in female rats exposed to N-methyl-2-pyrrolidone. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2012, 95, 195–201. [Google Scholar] [CrossRef]
- Sitarek, K.; Stetkiewicz, J. Assessment of reproductive toxicity and gonadotoxic potential of N-methyl-2-pyrrolidone in male rats. Int. J. Occup. Med. Environ. Health 2008, 21, 73–80. [Google Scholar] [CrossRef]
- Erakca, M.; Baumann, M.; Bauer, W.; de Biasi, L.; Hofmann, J.; Bold, B.; Weil, M. Energy flow analysis of laboratory scale lithium-ion battery cell production. iScience 2021, 24, 102437. [Google Scholar] [CrossRef]
- Pettinger, K.H.; Dong, W. When does the operation of a battery become environmentally positive? J. Electrochem. Soc. 2016, 164, A6274. [Google Scholar] [CrossRef]
- Yuan, C.; Deng, Y.; Li, T.; Yang, F. Manufacturing energy analysis of lithium ion battery pack for electric vehicles. CIRP Ann. 2017, 66, 53–56. [Google Scholar] [CrossRef]
- Ahmed, S.; Nelson, P.A.; Gallagher, K.G.; Dees, D.W. Energy impact of cathode drying and solvent recovery during lithium-ion battery manufacturing. J. Power Sources 2016, 322, 169–178. [Google Scholar] [CrossRef]
- Guerfi, A.; Kaneko, M.; Petitclerc, M.; Mori, M.; Zaghib, K. LiFePO4 water-soluble binder electrode for Li-ion batteries. J. Power Sources 2007, 163, 1047–1052. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Akbulut, A.; Guler, M.O.; Akbulut, H. A different method for producing a flexible LiMn2O4/MWCNT composite electrode for lithium-ion batteries. J. Appl. Electrochem. 2014, 44, 209–214. [Google Scholar] [CrossRef]
- Huttner, F.; Haselrieder, W.; Kwade, A. The influence of different post-drying procedures on remaining water content and physical and electrochemical properties of lithium-ion batteries. Energy Technol. 2020, 8, 1900245. [Google Scholar] [CrossRef]
- Bryntesen, S.N.; Kahrom, A.; Lamb, J.J.; Tolstorebrov, I.; Burheim, O.S. Experimental Analysis of Drying Kinetics and Quality Aspects of Convection-Dried Cathodes at Laboratory Scale. Batteries 2023, 9, 96. [Google Scholar] [CrossRef]
- Bryntesen, S.N.; Strømman, A.H.; Tolstorebrov, I.; Shearing, P.R.; Lamb, J.J.; Stokke Burheim, O. Opportunities for the state-of-the-art production of LiB electrodes—A review. Energies 2021, 14, 1406. [Google Scholar] [CrossRef]
- Rollag, K.; Juarez-Robles, D.; Du, Z.; Wood, D.L., III; Mukherjee, P.P. Drying temperature and capillarity-driven crack formation in aqueous processing of Li-ion battery electrodes. ACS Appl. Energy Mater. 2019, 2, 4464–4476. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.M.; Li, J.; An, S.J.; Wood, M.; Sheng, Y.; Wood, D.L., III. Enabling aqueous processing for crack-free thick electrodes. J. Power Sources 2017, 354, 200–206. [Google Scholar] [CrossRef]
- Marks, T.; Trussler, S.; Smith, A.J.; Xiong, D.; Dahn, J.R. A guide to Li-ion coin-cell electrode making for academic researchers. J. Electrochem. Soc. 2010, 158, A51. [Google Scholar] [CrossRef]
- Jaiser, S.; Müller, M.; Baunach, M.; Bauer, W.; Scharfer, P.; Schabel, W. Investigation of film solidification and binder migration during drying of Li-Ion battery anodes. J. Power Sources 2016, 318, 210–219. [Google Scholar] [CrossRef]
- Stein, M.; Mistry, A.; Mukherjee, P.P. Mechanistic understanding of the role of evaporation in electrode processing. J. Electrochem. Soc. 2017, 164, A1616. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.Z.; Yuan, H.; Hu, J.K.; Huang, J.Q.; Zhang, Q. Dry electrode technology, the rising star in solid-state battery industrialization. Matter 2022, 5, 876–898. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Wang, Z.; Xu, J.; Ma, T.; Chen, L.; Wu, F. Progress in solvent-free dry-film technology for batteries and supercapacitors. Mater. Today 2022, 55, 92–109. [Google Scholar] [CrossRef]
- Hood, Z.D.; Mane, A.U.; Sundar, A.; Tepavcevic, S.; Zapol, P.; Eze, U.D.; Connell, J.G. Multifunctional Coatings on Sulfide-Based Solid Electrolyte Powders with Enhanced Processability, Stability, and Performance for Solid-State Batteries. Adv. Mater. 2023, 35, 2300673. [Google Scholar] [CrossRef] [PubMed]
- Karabelli, D.; Birke, K.P.; Weeber, M. A performance and cost overview of selected solid-state electrolytes: Race between polymer electrolytes and inorganic sulfide electrolytes. Batteries 2021, 7, 18. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.; Char, K.; Kim, K.J.; Choi, J.W. Issues and advances in scaling up sulfide-based all-solid-state batteries. Acc. Chem. Res. 2021, 54, 3390–3402. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Bockholt, H.; Haselrieder, W.; Kwade, A. Discontinuous and continuous processing of low-solvent battery slurries for lithium nickel cobalt manganese oxide electrodes. J. Electron. Mater. 2015, 44, 4434–4443. [Google Scholar] [CrossRef]
- Lopez, J.; Pletscher, S.; Aemissegger, A.; Bucher, C.; Gallou, F. N-butylpyrrolidinone as an alternative solvent for solid-phase peptide synthesis. Org. Process Res. Dev. 2018, 22, 494–503. [Google Scholar] [CrossRef]
- Voropaeva, D.; Novikova, S.; Xu, T.; Yaroslavtsev, A. Polymer electrolytes for LIBs based on perfluorinated sulfocationic Nepem-117 membrane and aprotic solvents. J. Phys. Chem. B 2019, 123, 10217–10223. [Google Scholar] [CrossRef]
- Zhou, C.; Bag, S.; Lv, B.; Thangadurai, V. Understanding the role of solvents on the morphological structure and Li-ion conductivity of poly(vinylidene fluoride)-based polymer electrolytes. J. Electrochem. Soc. 2020, 167, 070552. [Google Scholar] [CrossRef]
- Dolzhenko, A.V. Ethyl lactate and its aqueous solutions as sustainable media for organic synthesis. Sustain. Chem. Pharm. 2020, 18, 100322. [Google Scholar] [CrossRef]
- Sarkar, A.; May, R.; Valmonte, Z.; Marbella, L.E. PolarClean & dimethyl isosorbide: Green matches in formulating cathode slurry. Energy Adv. 2022, 1, 671–676. [Google Scholar]
- Bottino, A.; Capannelli, G.; Munari, S.; Turturro, A. Solubility parameters of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1988, 26, 785–794. [Google Scholar] [CrossRef]
- Pastore, B.M.; Savelski, M.J.; Slater, C.S.; Richetti, F.A. Life cycle assessment of N-methyl-2-pyrrolidone reduction strategies in the manufacture of resin precursors. Clean Technol. Environ. Policy 2016, 18, 2635–2647. [Google Scholar]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage—The transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Doberdò, I.; Löffler, N.; Laszczynski, N.; Cericola, D.; Penazzi, N.; Bodoardo, S.; Passerini, S. Enabling aqueous binders for lithium battery cathodes Carbon coating of aluminum current collector. J. Power Sources 2014, 248, 1000–1006. [Google Scholar] [CrossRef]
- Wood, D.L., III; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium-ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Hofmann, M.; Kapuschinski, M.; Guntow, U.; Giffin, G.A. Implications of aqueous processing for high energy density cathode materials: Part I. Ni-rich layered oxides. J. Electrochem. Soc. 2020, 167, 140512. [Google Scholar] [CrossRef]
- Li, C.C.; Lin, Y.S. Interactions between organic additives and active powders in water-based lithium iron phosphate electrode slurries. J. Power Sources 2012, 220, 413–421. [Google Scholar] [CrossRef]
- Li, J.; Daniel, C.; Wood, D. Materials processing for lithium-ion batteries. J. Power Sources 2011, 196, 2452–2460. [Google Scholar] [CrossRef]
- Kuenzel, M.; Bresser, D.; Diemant, T.; Carvalho, D.V.; Kim, G.T.; Behm, R.J.; Passerini, S. Complementary strategies toward the aqueous processing of high-voltage LiNi0.5Mn1.5O4 lithium-ion cathodes. ChemSusChem 2018, 11, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Z.; Ruther, R.E.; An, S.J.; David, L.A.; Hays, K.; Wood, D.L. Toward low-cost, high-energy density, and high-power density lithium-ion batteries. JOM 2017, 69, 1484–1496. [Google Scholar] [CrossRef]
- Radloff, S.; Carbonari, G.; Scurtu, R.G.; Hölzle, M.; Wohlfahrt-Mehrens, M. Advances in the Aqueous Processing of Ni-Rich Positive Electrodes. Electrochem. Soc. Meet. Abstr. 2022, 242, 284. [Google Scholar] [CrossRef]
- Bauer, W.; Çetinel, F.A.; Müller, M.; Kaufmann, U. Effects of pH control by acid addition at the aqueous processing of cathodes for lithium-ion batteries. Electrochim. Acta 2019, 317, 112–119. [Google Scholar] [CrossRef]
- Loeffler, N.; Kim, G.T.; Mueller, F.; Diemant, T.; Kim, J.K.; Behm, R.J.; Passerini, S. In situ coating of Li[Ni0.33Mn0.33Co0.33]O2 particles to enable aqueous electrode processing. ChemSusChem 2016, 9, 1112–1117. [Google Scholar] [CrossRef]
- Carvalho, D.V.; Loeffler, N.; Kim, G.T.; Marinaro, M.; Wohlfahrt-Mehrens, M.; Passerini, S. Study of water-based lithium titanate electrode processing: The role of pH and binder molecular structure. Polymers 2016, 8, 276. [Google Scholar] [CrossRef]
- Bichon, M.; Sotta, D.; Dupré, N.; De Vito, E.; Boulineau, A.; Porcher, W.; Lestriez, B. Study of immersion of LiNi0.5Mn0.3Co0.2O2 material in water for aqueous processing of positive electrode for Li-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 18331–18341. [Google Scholar] [CrossRef]
- Belboom, S.; Szöcs, C.; Léonard, A. Environmental impacts of phosphoric acid production using di-hemihydrate process: A Belgian case study. J. Clean. Prod. 2015, 108, 978–986. [Google Scholar] [CrossRef]
- Liu, J.; Xu, C.; Chen, Z.; Ni, S.; Shen, Z.X. Progress in aqueous rechargeable batteries. Green Energy Environ. 2018, 3, 20–41. [Google Scholar] [CrossRef]
- Chen, L.; Cao, L.; Ji, X.; Hou, S.; Li, Q.; Chen, J.; Yang, C.; Eidson, N.; Wang, C. Enabling safe aqueous lithium-ion open batteries by suppressing the oxygen reduction reaction. Nat. Commun. 2020, 11, 2638. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, L.; Qu, S.; Deng, D.; Wu, Y.; Yan, X.; Peng, D. Promising electrode and electrolyte materials for high-energy-density thin-film lithium batteries. Energy Environ. Mater. 2022, 5, 133–156. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Neudecker, B.; Ueda, A.; Evans, C.D. Thin-film lithium and lithium-ion batteries. Solid State Ion. 2000, 135, 33–45. [Google Scholar] [CrossRef]
- Chiu, K.F. Lithium cobalt oxide thin films deposited at low temperature by ionized magnetron sputtering. Thin Solid Films 2007, 515, 4614–4618. [Google Scholar] [CrossRef]
- Patil, A.; Patil, V.; Shin, D.W.; Choi, J.W.; Paik, D.S.; Yoon, S.J. Issues and challenges facing rechargeable thin-film lithium batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Reyes Jiménez, A.; Klöpsch, R.; Wagner, R.; Rodehorst, U.C.; Kolek, M.; Nöolle, R.; Placke, T. A step toward high-energy silicon-based thin film lithium-ion batteries. ACS Nano 2017, 11, 4731–4744. [Google Scholar] [CrossRef]
- Maximov, M.; Nazarov, D.; Rumyantsev, A.; Koshtyal, Y.; Ezhov, I.; Mitrofanov, I.; Popovich, A. Atomic layer deposition of lithium–nickel–silicon oxide cathode material for thin-film lithium-ion batteries. Energies 2020, 13, 2345. [Google Scholar] [CrossRef]
- Maurel, A.; Grugeon, S.; Armand, M.; Fleutot, B.; Courty, M.; Prashantha, K.; Dupont, L. Overview on lithium-ion battery 3D-printing by means of material extrusion. ECS Trans. 2020, 98, 3. [Google Scholar] [CrossRef]
- Fu, K.; Wang, Y.; Yan, C.; Yao, Y.; Chen, Y.; Dai, J.; Hu, L. Graphene oxide-based electrode inks for 3D-printed lithium-ion batteries. Adv. Mater. 2016, 28, 2587–2594. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D printing of interdigitated Li-Ion microbattery architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.W.; Down, M.P.; Zhang, Y.; Ji, X.; Rowley-Neale, S.J.; Smith, G.C.; Banks, C.E. 3D printed graphene-based energy storage devices. Sci. Rep. 2017, 7, 42233. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.; Somogyi, R.; Niu, S.; Cruz, M.A.; Yang, F.; Catenacci, M.J.; Wiley, B.J. Three-dimensional printing of a complete lithium-ion battery with fused filament fabrication. ACS Appl. Energy Mater. 2018, 1, 5268–5279. [Google Scholar] [CrossRef]
- Trembacki, B.; Duoss, E.; Oxberry, G.; Stadermann, M.; Murthy, J. Mesoscale electrochemical performance simulation of 3D interpenetrating lithium-ion battery electrodes. J. Electrochem. Soc. 2019, 166, A923. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Hu, L. 3D-printed all-fiber Li-ion battery toward wearable energy storage. Adv. Funct. Mater. 2017, 27, 1703140. [Google Scholar] [CrossRef]
- Yang, K.; Xie, X.; Du, X.; Zuo, Y.; Zhang, Y. Research on Micromechanical Behavior of Current Collector of Lithium-Ion Batteries Battery Cathode during the Calendering Process. Processes 2023, 11, 1800. [Google Scholar] [CrossRef]
- Schmitt, M. Slot Die Coating of Lithium-Ion Battery Electrodes; KIT Scientific Publishing: Karlsruhe, Germany, 2016. [Google Scholar]
- Schmitt, M.; Baunach, M.; Wengeler, L.; Peters, K.; Junges, P.; Scharfer, P.; Schabel, W. Slot-die processing of lithium-ion battery electrodes—Coating window characterization. Chem. Eng. Process. 2013, 68, 32–37. [Google Scholar] [CrossRef]
- Schmitt, M.; Scharfer, P.; Schabel, W. Slot die coating of lithium-ion battery electrodes: Investigations on edge effect issues for stripe and pattern coatings. J. Coat. Technol. Res. 2014, 11, 57–63. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for lithium insertion in carbonaceous materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Guyomard, D.; Tarascon, J.M. Rechargeable Li1+xMn2O4/Carbon cells with a new electrolyte composition: Potentiostatic studies and application to practical cells. J. Electrochem. Soc. 1993, 140, 3071. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, A.; Lee, J.Y. Cycle life improvement of LiMn2O4 cathode in rechargeable lithium batteries. J. Power Sources 1998, 74, 228–233. [Google Scholar] [CrossRef]
- Chen, J.M.; Tsai, C.L.; Yao, C.Y.; Sheu, S.P.; Shih, H.C. Experimental design method applied to Li/LiCoO2 rechargeable cells. Mater. Chem. Phys. 1997, 51, 190–194. [Google Scholar] [CrossRef]
- Wentker, M.; Greenwood, M.; Leker, J. A bottom-up approach to lithium-ion battery cost modeling with a focus on cathode active materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Kiemel, S.; Glöser-Chahoud, S.; Waltersmann, L.; Schutzbach, M.; Sauer, A.; Miehe, R. Assessing the application-specific substitutability of lithium-ion battery cathode chemistries based on material criticality, performance, and price. Resources 2021, 10, 87. [Google Scholar] [CrossRef]
- Torres-Rodríguez, J.S. Reversing Planar Gliding, Microcracking Enhances Performance of Single-Crystalline Ni-Rich Cathode. MRS Bull. 2021, 46, 560. [Google Scholar] [CrossRef]
- Yi, M.; Li, J.; Fan, X.; Bai, M.; Zhang, Z.; Hong, B.; Lai, Y. Single crystal Ni-rich layered cathodes enabling superior performance in all-solid-state batteries with PEO-based solid electrolytes. J. Mater. Chem. A 2021, 9, 16787–16797. [Google Scholar] [CrossRef]
- Trevisanello, E.; Ruess, R.; Conforto, G.; Richter, F.H.; Janek, J. Polycrystalline and single crystalline NCM cathode materials—Quantifying particle cracking, active surface area, and lithium diffusion. Adv. Energy Mater. 2021, 11, 2003400. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Guo, C.; Liu, H.; Bao, W.; Li, J.; Wang, F. Spinel LiMn2O4 cathode materials in wide voltage window: Single-crystalline versus polycrystalline. Crystals 2022, 12, 317. [Google Scholar] [CrossRef]
- He, S.; Mian, J. The Progress of Carbon Coating Modification on the Surface of Lithium Iron Phosphate Cathode Materials. Highlights Sci. Eng. Technol. 2022, 3, 43–49. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent development in carbon-LiFePO4 cathodes for lithium-ion batteries: A mini review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From materials to cell: State-of-the-art and prospective technologies for lithium-ion battery electrode processing. Chem. Rev. 2021, 122, 903–956. [Google Scholar] [CrossRef]
- Chu, B.; Guo, Y.J.; Shi, J.L.; Yin, Y.X.; Huang, T.; Su, H.; Yu, A.; Guo, Y.G.; Li, Y. Cobalt in high-energy-density layered cathode materials for lithium ion batteries. J. Power Sources 2022, 544, 231873. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Sun, H.H.; Ryu, H.H.; Kim, U.H.; Weeks, J.A.; Heller, A.; Sun, Y.K.; Mullins, C.B. Beyond doping and coating: Prospective strategies for stable high-capacity layered Ni-rich cathodes. ACS Energy Lett. 2020, 5, 1136–1146. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, J.S.; Fu, Y.Z. Recent strategies for improving the performances of rechargeable lithium batteries with sulfur-and oxygen-based conversion cathodes. Energy Mater. 2023, 3, 300015. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Sun, X. Towards high-performance solid-state Li–S batteries: From fundamental understanding to engineering design. Chem. Soc. Rev. 2020, 49, 2140–2195. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, H.; Cui, Y. Formulating energy density for designing practical lithium–sulfur batteries. Nat. Energy 2022, 7, 312–319. [Google Scholar] [CrossRef]
- Zhu, J.; Zou, J.; Cheng, H.; Gu, Y.; Lu, Z. High energy batteries based on sulfur cathode. Green Energy Environ. 2019, 4, 345–359. [Google Scholar] [CrossRef]
- Lee, J.; Ko, B.; Kang, J.; Chung, Y.; Kim, Y.; Halim, W.; Lee, J.H.; Joo, Y.L. Facile and scalable fabrication of highly loaded sulfur cathodes and lithium–sulfur pouch cells via air-controlled electrospray. Mater. Today Energy 2017, 6, 255–263. [Google Scholar] [CrossRef]
- Liu, Y.T.; Liu, S.; Li, G.R.; Yan, T.Y.; Gao, X.P. High volumetric energy density sulfur cathode with heavy and catalytic metal oxide host for lithium–sulfur battery. Adv. Sci. 2020, 7, 1903693. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-energy batteries: Beyond lithium-ion and their long road to commercialisation. Nano-Micro Lett. 2022, 14, 94. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, L.; Guo, C.; Li, D.; Vasileff, A.; Wang, H.; Qiao, S.Z. A 3D hybrid of chemically coupled nickel sulfide and hollow carbon spheres for high performance lithium–sulfur batteries. Adv. Funct. Mater. 2017, 27, 1702524. [Google Scholar] [CrossRef]
- Wei Seh, Z.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.C.; Cui, Y. Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, N.; Zhou, W.; Zhang, H.; Li, X.; Zhang, H. Sulfur embedded in one-dimensional French fries-like hierarchical porous carbon derived from a metal–organic framework for high performance lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 15314–15323. [Google Scholar] [CrossRef]
- Das, D.; Manna, S.; Puravankara, S. Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review. Batteries 2023, 9, 193. [Google Scholar] [CrossRef]
- Muralidharan, N.; Essehli, R.; Hermann, R.P.; Amin, R.; Jafta, C.; Zhang, J.; Belharouak, I. Lithium Iron Aluminum Nickelate, LiNixFeyAlzO2—New Sustainable Cathodes for Next-Generation Cobalt-Free Li-Ion Batteries. Adv. Mater. 2020, 32, 2002960. [Google Scholar] [CrossRef]
- Sim, R.; Lee, S.; Li, W.; Manthiram, A. Influence of calendering on the electrochemical performance of LiNi0.9Mn0.05Al0.05O2 cathodes in lithium-ion cells. ACS Appl. Mater. Interfaces 2021, 13, 42898–42908. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.; Manthiram, A. High-nickel NMA: A cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries. Adv. Mater. 2020, 32, 2002718. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Ruther, R.E.; Du, Z.; Self, E.C.; Meyer, H.M., III; Deniel, C.; Belharouak, I.; Wood, D.L., III. Chemical stability and long-term cell performance of low-cobalt, Ni-Rich cathodes prepared by aqueous processing for high-energy Li-Ion batteries. Energy Storage Mater. 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N. A perspective on the sustainability of cathode materials used in lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2102028. [Google Scholar] [CrossRef]
- Zeng, W.; Xia, F.; Tian, W.; Cao, F.; Chen, J.; Wu, J.; Song, R.; Mu, S. Single-crystal high-nickel layered cathodes for lithium-ion batteries: Advantages, mechanism, challenges and approaches. Curr. Opin. Electrochem. 2022, 31, 100831. [Google Scholar] [CrossRef]
- Langdon, J.; Manthiram, A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 37, 143–160. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Zhang, H. Lithium-ion batteries–Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Takada, K.; Inada, T.; Kajiyama, A.; Sasaki, H.; Kondo, S.; Watanabe, M.; Murayama, M.; Kanno, R. Solid-state lithium battery with graphite anode. Solid State Ion. 2003, 158, 269–274. [Google Scholar] [CrossRef]
- Goldman, A.R.; Rotondo, F.S.; Swallow, J.G. Lithium ion Battery Industrial Base in the US and Abroad; Institute for Defense Analyses: Alexandria, VA, USA, 2019; p. 67. [Google Scholar]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material–fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Ghanooni Ahmadabadi, V.; Rahman, M.M.; Chen, Y. A Study on High-Rate Performance of Graphite Nanostructures Produced by Ball Milling as Anode for Lithium-Ion Batteries. Micromachines 2023, 14, 191. [Google Scholar] [CrossRef]
- Franco Gonzalez, A.; Yang, N.H.; Liu, R.S. Silicon anode design for lithium-ion batteries: Progress and perspectives. J. Phys. Chem. C 2017, 121, 27775–27787. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Li, M.; Gu, J.; Feng, X.; He, H.; Zeng, C. Amorphous-silicon@ silicon oxide/chromium/carbon as an anode for lithium-ion batteries with excellent cyclic stability. Electrochim. Acta 2015, 164, 163–170. [Google Scholar] [CrossRef]
- Huo, H.; Janek, J. Silicon as emerging anode in solid-state batteries. ACS Energy Lett. 2022, 7, 4005–4016. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, H.; Liu, W.; Lee, H.W.; Yan, K.; Zhuo, D.; Lin, D.; Liu, N.; Cui, Y. Artificial solid electrolyte interphase-protected Li x Si nanoparticles: An efficient and stable prelithiation reagent for lithium-ion batteries. J. Am. Chem. Soc. 2015, 137, 8372–8375. [Google Scholar] [CrossRef] [PubMed]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, J.; Zhang, Q.; Su, X. Recent advancement of SiOx based anodes for lithium-ion batteries. J. Power Sources 2017, 363, 126–144. [Google Scholar] [CrossRef]
- Tzeng, Y.; Chen, R.; He, J.L. Silicon-based anode of lithium ion battery made of nano silicon flakes partially encapsulated by silicon dioxide. Nanomaterials 2020, 10, 2467. [Google Scholar] [CrossRef]
- Li, Z.; Du, M.; Guo, X.; Zhang, D.; Wang, Q.; Sun, H.; Wang, B.; Wu, Y.A. Research progress of SiOx-based anode materials for lithium-ion batteries. Chem. Eng. J. 2023, 473, 145294. [Google Scholar] [CrossRef]
- Sun, M.; Ma, J.; Xu, M.; Yang, H.; Zhang, J.; Wang, C. A Low-Cost SiOx/C@ Graphite Composite Derived from Oat Husk as an Advanced Anode for High-Performance Lithium-Ion Batteries. ACS Omega 2022, 7, 15123–15131. [Google Scholar] [CrossRef]
- Kirkaldy, N.; Samieian, M.A.; Offer, G.J.; Marinescu, M.; Patel, Y. Lithium-Ion Battery Degradation: Measuring Rapid Loss of Active Silicon in Silicon–Graphite Composite Electrodes. ACS Appl. Energy Mater. 2022, 5, 13367–13376. [Google Scholar] [CrossRef]
- Andersen, H.F.; Foss, C.E.L.; Voje, J.; Tronstad, R.; Mokkelbost, T.; Vullum, P.E.; Ulvestad, A.; Kirkengen, M.; Mæhlen, J.P. Silicon-Carbon Composite Anodes from Industrial Battery Grade Silicon. Sci. Rep. 2019, 9, 14814. [Google Scholar] [CrossRef]
- Hays, K.A.; Ruther, R.E.; Kukay, A.J.; Cao, P.; Saito, T.; Wood, D.L., III; Li, J. What Makes Lithium Substituted Polyacrylic Acid a Better Binder than Polyacrylic Acid for Silicon-Graphite Composite Anodes? J. Power Sources 2018, 384, 136–144. [Google Scholar] [CrossRef]
- Ratyński, M.; Hamankiewicz, B.; Krajewski, M.; Boczar, M.; Czerwiński, A. The Effect of Compressive Stresses on a Silicon Electrode’s Cycle Life in a Li-Ion Battery. RSC Adv. 2018, 8, 22546–22551. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, H.C.; Jung, H.; Wakita, S.; Cho, S.; Yoon, J.; Lee, J.; Ueda, A.; Choi, B.; Han, I.T.; et al. Interplay between Electrochemical Reactions and Mechanical Responses in Silicon–Graphite Anodes and Its Impact on Degradation. Nat. Commun. 2021, 12, 2714. [Google Scholar] [CrossRef]
- Minseong, K.O.; Sujong, C.H.A.E.; Jaephil, C.H.O. Challenges in Accommodating Volume Change of Si Anodes for Li-ion Batteries. ChemElectroChem 2015, 2, 1645–1651. [Google Scholar]
- Wang, H.; Lu, S.H.; Wang, X.; Xia, S.; Chew, H.B. A Review of the Multiscale Mechanics of Silicon Electrodes in High-Capacity Lithium-Ion Batteries. J. Phys. D Appl. Phys. 2021, 55, 063001. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based Anodes for Lithium-Ion Batteries: Effectiveness of Materials Synthesis and Electrode Preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Lithium Titanate as Anode Material for Lithium-Ion Cells: A Review. Ionics 2014, 20, 601–620. [Google Scholar] [CrossRef]
- Zaghib, K.; Simoneau, M.; Armand, M.; Gauthier, M. Electrochemical Study of Li4Ti5O12 as Negative Electrode for Li-ion Polymer Rechargeable Batteries. J. Power Sources 1999, 81, 300–305. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Kalogiannis, T.; He, J.; Patil, M.S.; Muller, J.D.; Berecibar, M. Advanced Hybrid Thermal Management System for LTO Battery Module Under Fast Charging. Case Stud. Therm. Eng. 2022, 33, 101938. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Fang, S.; Tang, Y. Synthesis of Sawtooth-like Li4Ti5O12 Nanosheets as Anode Materials for Li-ion Batteries. Electrochim. Acta 2010, 55, 6596–6600. [Google Scholar] [CrossRef]
- Jin, X.; Han, Y.; Zhang, Z.; Chen, Y.; Li, J.; Yang, T.; Wang, X.; Li, W.; Han, X.; Jiao, S.; et al. Mesoporous Single-Crystal Lithium Titanate Enabling Fast-Charging Li-Ion Batteries. Adv. Mater. 2022, 34, 2109356. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, L.; Fang, S.; Qiu, Z. Li4Ti5O12 Hollow Microspheres Assembled by Nanosheets as an Anode Material for High-Rate Lithium-Ion Batteries. Electrochim. Acta 2009, 54, 6244–6249. [Google Scholar] [CrossRef]
- Qasim, K.F.; Mousa, M.A. Physicochemical Properties of Oriented Crystalline Assembled Polyaniline/Metal Doped Li4Ti5O12 Composites for Li-ion Storage. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2601–2617. [Google Scholar] [CrossRef]
- Wen, Z.; Huang, S.; Yang, X.; Lin, B. High Rate Electrode Materials for Lithium Ion Batteries. Solid State Ion. 2008, 179, 1800–1805. [Google Scholar] [CrossRef]
- Ouyang, C.Y.; Zhong, Z.Y.; Lei, M.S. Ab Initio Studies of Structural and Electronic Properties of Li4Ti5O12 Spinel. Electrochem. Commun. 2007, 9, 1107–1112. [Google Scholar] [CrossRef]
- Sun, X.; Radovanovic, P.V.; Cui, B. Advances in Spinel Li4Ti5O12 Anode Materials for Lithium-Ion Batteries. New J. Chem. 2015, 39, 38–63. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, L.; Zhang, L.; Wang, Q.; Yan, H.; Zhao, B.; An, H. Electrochemical and Transport Properties of Te-Doped Li4Ti5O12 as Anode Material for Lithium-Ion Half/Full Batteries. J. Alloys Compd. 2022, 897, 162744. [Google Scholar] [CrossRef]
- Annisa, N.; Orlando, I.; Syahrial, A.Z. Effect of Activated Carbon Addition on Electrochemical Performance of Li4Ti5O12/Nano Si Composite as Anode Material for LiB. IOP Conf. Ser. Mater. Sci. Eng. 2019, 553, 012057. [Google Scholar] [CrossRef]
- Chen, C.; Agrawal, R.; Wang, C. High Performance Li4Ti5O12/Si Composite Anodes for Li-ion Batteries. Nanomaterials 2015, 5, 1469–1480. [Google Scholar] [CrossRef]
- Selinis, P.; Farmakis, F. A Review on the Anode and Cathode Materials for Lithium-Ion Batteries with Improved Subzero Temperature Performance. J. Electrochem. Soc. 2022, 169, 010526. [Google Scholar] [CrossRef]
- Marinow, A.; Katcharava, Z.; Binder, W.H. Self-Healing Polymer Electrolytes for Next-Generation Lithium Batteries. Polymers 2023, 15, 1145. [Google Scholar] [CrossRef] [PubMed]
- Peled, E.; Menkin, S. SEI: Past, Present, and Future. J. Electrochem. Soc. 2017, 164, A1703. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in Electrolytes for Rechargeable Li-Based Batteries and Beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Santos, D.M. Applications of Polymer Electrolytes in Lithium-Ion Batteries: A Review. Polymers 2023, 15, 3907. [Google Scholar] [CrossRef] [PubMed]
- Salado, M.; Lizundia, E. Advances, Challenges, and Environmental Impacts in Metal–Air Battery Electrolytes. Mater. Today Energy 2022, 28, 101064. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Méndez, S. Toward Sustainable Solid Polymer Electrolytes for Lithium-Ion Batteries. ACS Omega 2022, 7, 14457–14464. [Google Scholar] [CrossRef]
- Hall, D.S.; Self, J.; Dahn, J.R. Dielectric Constants for Quantum Chemistry and Li-Ion Batteries: Solvent Blends of Ethylene Carbonate and Ethyl Methyl Carbonate. J. Phys. Chem. C 2015, 119, 22322–22330. [Google Scholar] [CrossRef]
- Lv, W.; Zhu, C.; Chen, J.; Ou, C.; Zhang, Q.; Zhong, S. High Performance of Low-Temperature Electrolyte for Lithium-Ion Batteries Using Mixed Additives. Chem. Eng. J. 2021, 418, 129400. [Google Scholar] [CrossRef]
- Bushkova, O.V.; Yaroslavtseva, T.V.; Dobrovolsky, Y.A. New Lithium Salts in Electrolytes for Lithium-Ion Batteries. Russ. J. Electrochem. 2017, 53, 677–699. [Google Scholar] [CrossRef]
- Younesi, R.; Veith, G.M.; Johansson, P.; Edström, K.; Vegge, T. Lithium Salts for Advanced Lithium Batteries: Li–Metal, Li–O2, and Li–S. Energy Environ. Sci. 2015, 8, 1905–1922. [Google Scholar] [CrossRef]
- Sharova, V. Enhancing the Performance of Lithium Batteries through the Development of Improved Electrolyte Formulation, Formation Protocol, and Graphite Surface Modification. Ph.D. Dissertation, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 2017. [Google Scholar]
- Aurbach, D.; Markovsky, B.; Levi, M.D.; Levi, E.; Schechter, A.; Moshkovich, M.; Cohen, Y. New Insights into the Interactions Between Electrode Materials and Electrolyte Solutions for Advanced Nonaqueous Batteries. J. Power Sources 1999, 81, 95–111. [Google Scholar] [CrossRef]
- Bonilla, M.R.; Daza, F.A.G.; Cortés, H.A.; Carrasco, J.; Akhmatskaya, E. On the Interfacial Lithium Dynamics in Li7La3Zr2O12: Poly (ethylene oxide)(LiTFSI) Composite Polymer-Ceramic Solid Electrolytes Under Strong Polymer Phase Confinement. J. Colloid Interface Sci. 2022, 623, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, N.D.; Jayawardana, C.; Rynearson, L.; Hu, E.; Yang, X.Q.; Lucht, B.L. Use of Ethylene Carbonate Free Ester Solvent Systems with Alternative Lithium Salts for Improved Low-Temperature Performance in NCM622‖Graphite Li-ion Batteries. J. Electrochem. Soc. 2022, 169, 110504. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. Low-Temperature Performance of Li-ion Cells with a LiBF4-Based Electrolyte. J. Solid State Electrochem. 2003, 7, 147–151. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Study of LiBF4 as an Electrolyte Salt for a Li-ion Battery. J. Electrochem. Soc. 2002, 149, A586. [Google Scholar] [CrossRef]
- Ulihin, A.; Novozhilov, D.; Uvarov, N. Solid Electrolytes in the N-Propyl-N-methyl-pyrrolidinium Tetrafluoroborate—Lithium Tetrafluoroborate System. Batteries 2023, 9, 167. [Google Scholar] [CrossRef]
- Ndruru, S.T.C.L.; Wahyuningrum, D.; Bundjali, B.; Arcana, I.M. Preparation and Characterization of Biopolymer Electrolyte Membranes Based on LiClO4-Complexed Methyl Cellulose as Lithium-Ion Battery Separator. J. Eng. Technol. Sci. 2020, 52, 28–50. [Google Scholar] [CrossRef]
- Chen, H.P.; Fergus, J.W.; Jang, B.Z. The Effect of Ethylene Carbonate and Salt Concentration on the Conductivity of Propylene Carbonate| Lithium Perchlorate Electrolytes. J. Electrochem. Soc. 2000, 147, 399. [Google Scholar] [CrossRef]
- Maddu, A.; Sulaeman, A.S.; Wahyudi, S.T.; Rifai, A. Enhancing Ionic Conductivity of Carboxymethyl Cellulose-Lithium Perchlorate with Crosslinked Citric Acid as Solid Polymer Electrolytes for Lithium Polymer Batteries. Int. J. Renew. Energy Dev. 2022, 11. [Google Scholar] [CrossRef]
- Schmidt, M.; Heider, U.; Kuehner, A.; Oesten, R.; Jungnitz, M.; Ignat’Ev, N.; Sartori, P. Lithium Fluoroalkylphosphates: A New Class of Conducting Salts for Electrolytes for High Energy Lithium-Ion Batteries. J. Power Sources 2001, 97, 557–560. [Google Scholar] [CrossRef]
- Koch, V.R.; Goldman, J.L.; Mattos, C.J.; Mulvaney, M. Specular Lithium Deposits from Lithium Hexafluoroarsenate/Diethyl Ether Electrolytes. J. Electrochem. Soc. 1982, 129, 1. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Shechter, A.; Ein-Eli, Y.; Cohen, H. A Comparative Study of Synthetic Graphite and Li Electrodes in Electrolyte Solutions Based on Ethylene Carbonate-Dimethyl Carbonate Mixtures. J. Electrochem. Soc. 1996, 143, 3809. [Google Scholar] [CrossRef]
- Nazri, G.; Muller, R.H. Stability of Nonaqueous Electrolytes for Ambient Temperature Rechargeable Lithium Cells. Chem. Eng. Commun. 1985, 38, 383–391. [Google Scholar] [CrossRef]
- Zakiyyah, S.; Lestariningsih, T.; Rifai, A.; Ratri, C.; Sabrina, Q.; Subhan, A.; Slamet, P.; Muhammad, D.; Izha, M.Y. Modification of Lithium Source in LiBOB Salt of LiTFSI-LiBOB Electrolyte to Improve Lithium-Ion Battery Performance. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2022; Volume 2652. [Google Scholar]
- Liu, Z.; Chai, J.; Xu, G.; Wang, Q.; Cui, G. Functional Lithium Borate Salts and Their Potential Application in High-Performance Lithium Batteries. Coord. Chem. Rev. 2015, 292, 56–73. [Google Scholar] [CrossRef]
- Xue, Z.M.; Sun, B.B.; Zhou, W.; Chen, C.H. A New Lithium Salt with Dihydroxybenzene and Lithium Tetrafluoroborate for Lithium Battery Electrolytes. J. Power Sources 2011, 196, 8710–8713. [Google Scholar] [CrossRef]
- Azeez, F.; Fedkiw, P.S. Conductivity of LiBOB-Based Electrolyte for Lithium-Ion Batteries. J. Power Sources 2010, 195, 7627–7633. [Google Scholar] [CrossRef]
- Tan, S.; Ji, Y.J.; Zhang, Z.R.; Yang, Y. Recent Progress in Research on High-Voltage Electrolytes for Lithium-Ion Batteries. ChemPhysChem 2014, 15, 1956–1969. [Google Scholar] [CrossRef]
- Zhang, S.S.; Jow, T.R.; Amine, K.; Henriksen, G.L. LiPF6–EC–EMC Electrolyte for Li-ion Battery. J. Power Sources 2002, 107, 18–23. [Google Scholar] [CrossRef]
- Hayamizu, K. Direct Relations Between Ion Diffusion Constants and Ionic Conductivity for Lithium Electrolyte Solutions. Electrochim. Acta 2017, 254, 101–111. [Google Scholar] [CrossRef]
- Zhang, S.S. A Review on the Separators of Liquid Electrolyte Li-ion Batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.H.; Kim, J.H.; Lee, S.Y.; Lanceros-Méndez, S. Recent Advances on Separator Membranes for Lithium-Ion Battery Applications: From Porous Membranes to Solid Electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Heidari, A.A.; Mahdavi, H. Recent Development of Polyolefin-Based Microporous Separators for Li-Ion Batteries: A Review. Chem. Rec. 2020, 20, 570–595. [Google Scholar] [CrossRef] [PubMed]
- Kaimai, N.; Takita, K.; Kono, K.; Funaoka, H. Method of Producing Highly Permeable Microporous Polyolefin Membrane. U.S. Patent 6,153,133, 28 November 2000. [Google Scholar]
- Hashimoto, A.; Yagi, K.; Mantoku, H. Porous Film of High Molecular Weight Polyolefin and Process for Producing Same. U.S. Patent 6,048,607, 11 April 2000. [Google Scholar]
- Mahdavi, H.; Enayati Nook, M. Commercial, High-Impact Polypropylenes: Composition and Chain Structure as Revealed by Temperature-Gradient Extraction Fractionation. J. Appl. Polym. Sci. 2012, 125, 1606–1615. [Google Scholar] [CrossRef]
- Mahdavi, H.; Nook, M.E. Structure and Morphology of a Commercial High-Impact Polypropylene In-Reactor Alloy Synthesized Using a Spherical Ziegler–Natta Catalyst. Polymer Int. 2010, 59, 1701–1708. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, X.; Han, K.; Xiong, X. Natural Halloysite Nanotubes-Coated Polypropylene Membrane as Dual-Function Separator for Highly Safe Li-ion Batteries with Improved Cycling and Thermal Stability. Electrochim. Acta 2021, 379, 138182. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Ji, W.; Jiang, B.; Ai, F.; Yang, H.; Ai, X. Temperature-Responsive Microspheres-Coated Separator for Thermal Shutdown Protection of Lithium Ion Batteries. RSC Adv. 2015, 5, 172–176. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Zhang, Z. Separators for Lithium-Ion Batteries. In Lithium-Ion Batteries: Advanced Materials and Technologies; CRC Press: Boca Raton, FL, USA, 2011; Chapter 197. [Google Scholar]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and Performance Evaluation of Lithium-Ion Battery Separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef]
- Gor, G.Y.; Cannarella, J.; Leng, C.Z.; Vishnyakov, A.; Arnold, C.B. Swelling and Softening of Lithium-Ion Battery Separators in Electrolyte Solvents. J. Power Sources 2015, 294, 167–172. [Google Scholar] [CrossRef]
- Parikh, D.; Christensen, T.; Hsieh, C.T.; Li, J. Elucidation of Separator Effect on Energy Density of Li-ion Batteries. J. Electrochem. Soc. 2019, 166, A3377. [Google Scholar] [CrossRef]
- Peabody, C.; Arnold, C.B. The Role of Mechanically Induced Separator Creep in Lithium-Ion Battery Capacity Fade. J. Power Sources 2011, 196, 8147–8153. [Google Scholar] [CrossRef]
- Yang, M.; Hou, J. Membranes in Lithium Ion Batteries. Membranes 2012, 2, 367–383. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Chen, L.; Yang, P.; Zhao, J. Effect of a Thin Ceramic-Coating Layer on Thermal and Electrochemical Properties of Polyethylene Separator for Lithium-Ion Batteries. J. Power Sources 2014, 270, 547–553. [Google Scholar] [CrossRef]
- Lee, T.; Kim, W.K.; Lee, Y.; Ryou, M.H.; Lee, Y.M. Effect of Al2O3 Coatings Prepared by RF Sputtering on Polyethylene Separators for High-Power Lithium Ion Batteries. Macromol. Res. 2014, 22, 1190–1195. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Guo, B.; He, X. Effect of Al2O3/SiO2 Composite Ceramic Layers on Performance of Polypropylene Separator for Lithium-Ion Batteries. Ceram. Int. 2014, 40, 14105–14110. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Golodnitsky, D.; Strauss, E.; Peled, E.; Greenbaum, S. On Order and Disorder in Polymer Electrolytes. J. Electrochem. Soc. 2015, 162, A2551. [Google Scholar] [CrossRef]
- Zhang, X.; Sahraei, E.; Wang, K. Deformation and Failure Characteristics of Four Types of Lithium-Ion Battery Separators. J. Power Sources 2016, 327, 693–701. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Jeon, H.; Yeon, D.; Kim, B.H.; Cho, K.Y.; Lee, Y.M. In-depth Correlation of Separator Pore Structure and Electrochemical Performance in Lithium-Ion Batteries. J. Power Sources 2016, 325, 732–738. [Google Scholar] [CrossRef]
- Kalnaus, S.; Wang, Y.; Turner, J.A. Mechanical Behavior and Failure Mechanisms of Li-ion Battery Separators. J. Power Sources 2017, 348, 255–263. [Google Scholar] [CrossRef]
- Greene, J.P. Automotive Plastics and Composites: Materials and Processing; William Andrew: Norwich, UK, 2021. [Google Scholar]
- Barbosa, J.C.; Costa, C.M.; Lanceros-Méndez, S. Polymer-Based Separators for Lithium-Ion Batteries; In Nanomaterials for Electrochemical Energy Storage Devices; Wiley: Hoboken, NJ, USA, 2019; p. 429. [Google Scholar]
- Chang, W.; Dayong, W. LIB Separators and the Recent Technical Progress. Energy Storage Sci. Technol. 2016, 5, 120. [Google Scholar]
- Zhou, X.; Liao, Q.; Bai, T.; Yang, J. Nitrogen-Doped Microporous Carbon from Polyaspartic Acid Bonding Separator for High Performance Lithium-Sulfur Batteries. J. Electroanal. Chem. 2017, 791, 167–174. [Google Scholar] [CrossRef]
- Zhang, K.; Xiao, W.; Liu, J.; Yan, C. A Novel Self-Binding Composite Separator Based on Poly (Tetrafluoroethylene) Coating for Li-ion Batteries. Polymers 2018, 10, 1409. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Cui, G. Rigid–Flexible Coupling Polymer Electrolytes toward High-Energy Lithium Batteries. Macromol. Mater. Eng. 2018, 303, 1800337. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A Comprehensive Review on Fundamental Properties and Applications of Poly (Vinylidene Fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, J.H.; Park, M.S.; Kwon, H.K.; Kim, H.; Kim, Y.J. Enhancement of Electrochemical and Thermal Properties of Polyethylene Separators Coated with Polyvinylidene Fluoride–Hexafluoropropylene Co-Polymer for Li-ion Batteries. J. Power Sources 2012, 198, 298–302. [Google Scholar] [CrossRef]
- Sousa, R.E.; Kundu, M.; Gören, A.; Silva, M.M.; Liu, L.; Costa, C.M.; Lanceros-Mendez, S. Poly (vinylidene fluoride-co-chlorotrifluoroethylene)(PVDF-CTFE) Lithium-ion Battery Separator Membranes Prepared by Phase Inversion. RSC Adv. 2015, 5, 90428–90436. [Google Scholar] [CrossRef]
- Pan, J.L.; Zhang, Z.; Zhang, H.; Zhu, P.P.; Wei, J.C.; Cai, J.X.; Yang, Z.Y. Ultrathin and Strong Electrospun Porous Fiber Separator. ACS Appl. Energy Mater. 2018, 1, 4794–4803. [Google Scholar] [CrossRef]
- Othman, N.; Azahari, N.A.; Ismail, H. Thermal Properties of Polyvinyl Alcohol (PVOH)/Corn Starch Blend Film. Malays. Polym. J. 2011, 6, 147–154. [Google Scholar]
- Xiao, W.; Zhao, L.; Gong, Y.; Liu, J.; Yan, C. Preparation and Performance of Poly (Vinyl Alcohol) Porous Separator for Lithium-Ion Batteries. J. Membr. Sci. 2015, 487, 221–228. [Google Scholar] [CrossRef]
- Calleja, G.; Jourdan, A.; Ameduri, B.; Habas, J.P. Where is the Glass Transition Temperature of Poly (Tetrafluoroethylene)? A New Approach by Dynamic Rheometry and Mechanical Tests. Eur. Polym. J. 2013, 49, 2214–2222. [Google Scholar] [CrossRef]
- Xiong, M.; Tang, H.; Wang, Y.; Lin, Y.; Sun, M.; Yin, Z.; Pan, M. Expanded Polytetrafluoroethylene Reinforced Polyvinylidenefluoride–Hexafluoropropylene Separator with High Thermal Stability for Lithium-Ion Batteries. J. Power Sources 2013, 241, 203–211. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, J.S.; Min, J.; Lee, Y.H.; Choi, J.H.; Jang, M.C.; Choi, J.W. A Moisture-and Oxygen-Impermeable Separator for Aprotic Li-O2 Batteries. Adv. Funct. Mater. 2016, 26, 1747–1756. [Google Scholar] [CrossRef]
- Luo, H.F.; Qiao, Y.D. Preparation and Characterization of Cellulose Acetate-based Separator for Lithium-ion Batteries. J. Electrochem. 2017, 23, 9. [Google Scholar]
- Jeong, H.S.; Kim, J.H.; Lee, S.Y. A Novel Poly (Vinylidene Fluoride-Hexafluoropropylene)/Poly (Ethylene Terephthalate) Composite Nonwoven Separator with Phase Inversion-Controlled Microporous Structure for a Lithium-Ion Battery. J. Mater. Chem. 2010, 20, 9180–9186. [Google Scholar] [CrossRef]
- Choi, E.S.; Lee, S.Y. Particle size-dependent, tunable porous structure of a SiO2/poly (vinylidene fluoride-hexafluoropropylene)-coated poly (ethylene terephthalate) nonwoven composite separator for a lithium-ion battery. J. Mater. Chem. 2011, 21, 14747–14754. [Google Scholar] [CrossRef]
- Bierenbaum, H.S.; Isaacson, R.B.; Druin, M.L.; Plovan, S.G. Microporous polymeric films. Ind. Eng. Chem. Prod. Res. Dev. 1974, 13, 2–9. [Google Scholar] [CrossRef]
- Mun, S.C.; Won, J.H. Manufacturing processes of microporous polyolefin separators for lithium-ion batteries and correlations between mechanical and physical properties. Crystals 2021, 11, 1013. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, X.; Wen, J.; Wang, C.; Ma, X.; Yang, Y.; Huang, G.; Ye, H.M.; Xu, S. Research progress on high-temperature resistant polymer separators for lithium-ion batteries. Energy Storage Mater. 2022, 51, 638–659. [Google Scholar] [CrossRef]
- Takita, K.; Kono, K.; Takashima, T.; Okamoto, K. Microporous Polyolefin Membrane and Method of Producing Same. U.S. Patent 5,051,183, 24 September 1991. [Google Scholar]
- Yamada, K.; Nakamura, T. Microporous Membranes and Methods for Producing and Using Such Membranes, Assigned to Toray Tonen Specialty. U.S. Patent Application 20110117439A1, 19 May 2011. [Google Scholar]
- DeMeuse, M.T. Polymer-based Separators for Lithium-ion Batteries: Production, Processing, and Properties; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Wu, T.; Wang, K.; Xiang, M.; Fu, Q. Progresses in manufacturing techniques of lithium-ion battery separators in China. Chin. J. Chem. 2019, 37, 1207–1215. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, J. Electrode manufacturing for lithium-ion batteries—Analysis of current and next generation processing. J. Energy Storage 2019, 25, 100862. [Google Scholar] [CrossRef]
- Al-Shroofy, M.; Zhang, Q.; Xu, J.; Chen, T.; Kaur, A.P.; Cheng, Y.T. Solvent-free dry powder coating process for low-cost manufacturing of LiNi1/3Mn1/3Co1/3O2 cathodes in lithium-ion batteries. J. Power Sources 2017, 352, 187–193. [Google Scholar] [CrossRef]
- Wang, C.; Yu, R.; Duan, H.; Lu, Q.; Li, Q.; Adair, K.R.; Sun, X. Solvent-free approach for interweaving freestanding and ultrathin inorganic solid electrolyte membranes. ACS Energy Lett. 2021, 7, 410–416. [Google Scholar] [CrossRef]
- Aranmala, K.; Chanhaew, A.; Rahmawati, M.; Ikhsanudin, M.N.; Meethong, N. Effects of Conductive Agents on Electrochemical Performance of Water-Based LiNi0.6Mn0.2Co0.2O2 Cathodes for Cylindrical Cell Production of Lithium-Ion Batteries. Defect Diffus. Forum 2022, 417, 169–176. [Google Scholar] [CrossRef]
- Bi, J.; Du, Z.; Sun, J.; Liu, Y.; Wang, K.; Du, H.; Huang, W. On the Road to the Frontiers of Lithium-Ion Batteries: A Review and Outlook of Graphene Anodes. Adv. Mater. 2023, 35, 2210734. [Google Scholar] [CrossRef]
- Kirsch, D.J.; Lacey, S.D.; Kuang, Y.; Pastel, G.; Xie, H.; Connell, J.W.; Hu, L. Scalable dry processing of binder-free lithium-ion battery electrodes enabled by holey graphene. ACS Appl. Energy Mater. 2019, 2, 2990–2997. [Google Scholar] [CrossRef]
- Yan, Z.; Jiang, J.; Zhang, Y.; Yang, D.; Du, N. Scalable and low-cost synthesis of porous silicon nanoparticles as high-performance lithium-ion battery anode. Mater. Today Nano 2022, 18, 100175. [Google Scholar] [CrossRef]
- Chouchane, M.; Rucci, A.; Lombardo, T.; Ngandjong, A.C.; Franco, A.A. Lithium ion battery electrodes predicted from manufacturing simulations: Assessing the impact of the carbon-binder spatial location on the electrochemical performance. J. Power Sources 2019, 444, 227285. [Google Scholar] [CrossRef]
- Jaiser, S.; Funk, L.; Baunach, M.; Scharfer, P.; Schabel, W. Experimental investigation into battery electrode surfaces: The distribution of liquid at the surface and the emptying of pores during drying. J. Colloid Interface Sci. 2017, 494, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kallitsis, E.; Korre, A.; Kelsall, G.; Kupfersberger, M.; Nie, Z. Environmental life cycle assessment of the production in China of lithium-ion batteries with nickel-cobalt-manganese cathodes utilising novel electrode chemistries. J. Clean. Prod. 2020, 254, 120067. [Google Scholar] [CrossRef]
- Zanotto, F.M.; Dominguez, D.Z.; Ayerbe, E.; Boyano, I.; Burmeister, C.; Duquesnoy, M.; Franco, A.A. Data specifications for battery manufacturing digitalization: Current status, challenges, and opportunities. Batteries Supercaps 2022, 5, e202200224. [Google Scholar] [CrossRef]
- Wang, M.; Hu, J.; Wang, Y.; Cheng, Y.T. The influence of polyvinylidene fluoride (PVDF) binder properties on LiNi0.33Co0.33Mn0.33O2 (NMC) electrodes made by a dry-powder-coating process. J. Electrochem. Soc. 2019, 166, A2151. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Lou, F.; Yu, Z. Solvent-free lithium iron phosphate cathode fabrication with fibrillation of polytetrafluoroethylene. Electrochim. Acta 2023, 456, 142469. [Google Scholar] [CrossRef]
- Park, D.W.; Cañas, N.A.; Wagner, N.; Friedrich, K.A. Novel solvent-free direct coating process for battery electrodes and their electrochemical performance. J. Power Sources 2016, 306, 758–763. [Google Scholar] [CrossRef]
- Helmers, L.; Froböse, L.; Friedrich, K.; Steffens, M.; Kern, D.; Michalowski, P.; Kwade, A. Sustainable Solvent-Free Production and Resulting Performance of Polymer Electrolyte-Based All-Solid-State Battery Electrodes. Energy Technol. 2021, 9, 2000923. [Google Scholar] [CrossRef]
- Gyulai, A.; Bauer, W.; Ehrenberg, H. Dry Electrode Manufacturing in a Calender: The Role of Powder Premixing for Electrode Quality and Electrochemical Performance. ACS Appl. Energy Mater. 2023, 6, 5122–5134. [Google Scholar] [CrossRef]
- Tao, R.; Steinhoff, B.; Sawicki, C.H.; Sharma, J.; Sardo, K.; Bishtawi, A.; Li, J. Unraveling the impact of the degree of dry mixing on dry-processed lithium-ion battery electrodes. J. Power Sources 2023, 580, 233379. [Google Scholar] [CrossRef]
- Bauer, W.; Nötzel, D.; Wenzel, V.; Nirschl, H. Influence of dry mixing and distribution of conductive additives in cathodes for lithium ion batteries. J. Power Sources 2015, 288, 359–367. [Google Scholar] [CrossRef]
- Bockholt, H.; Haselrieder, W.; Kwade, A. Intensive powder mixing for dry dispersing of carbon black and its relevance for lithium-ion battery cathodes. Powder Technol. 2016, 297, 266–274. [Google Scholar] [CrossRef]
- Nam, Y.J.; Oh, D.Y.; Jung, S.H.; Jung, Y.S. Toward practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry-and slurry-mixing processes. J. Power Sources 2018, 375, 93–101. [Google Scholar] [CrossRef]
- Wenzel, V.; Nirschl, H.; Nötzel, D. Challenges in lithium-ion-battery slurry preparation and potential of modifying electrode structures by different mixing processes. Energy Technol. 2015, 3, 692–698. [Google Scholar] [CrossRef]
- Yang, J.; Sliva, A.; Banerjee, A.; Dave, R.N.; Pfeffer, R. Dry particle coating for improving the flowability of cohesive powders. Powder Technol. 2005, 158, 21–33. [Google Scholar] [CrossRef]
- Wenzel, V.; Moeller, R.S.; Nirschl, H. Influence of Mixing Technology and the Potential to Modify the Morphological Properties of Materials used in the Manufacture of Lithium-Ion Batteries. Energy Technol. 2014, 2, 176–182. [Google Scholar] [CrossRef]
- Mitchell, P.; Zhong, L.; Xi, X.; Zou, B. Dry Particle Based Adhesive and Dry Film and Methods of Making Same. U.S. Patent US20200152987A1, 14 May 2020. [Google Scholar]
- Zou, B.; Zhong, L.; Mitchell, P.; Xi, X. Dry-Particle Packaging Systems and Methods of Making Same. U.S. Patent US20060137158A1, 29 June 2006. [Google Scholar]
- Mitchell, P.; Zhong, L.; Xi, X. Recyclable Dry Particle Based Adhesive Electrode and Methods of Making Same. U.S. Patent US7342770B2, 11 March 2008. [Google Scholar]
- Wu, Y.; Wang, S.; Li, H.; Chen, L.; Wu, F. Progress in thermal stability of all-solid-state-Li-ion-batteries. InfoMat 2021, 3, 827–853. [Google Scholar] [CrossRef]
- Lu, P.; Liu, L.; Wang, S.; Xu, J.; Peng, J.; Yan, W.; Wang, Q.; Li, H.; Chen, L.; Wu, F. Superior all-solid-state batteries enabled by a gas-phase-synthesized sulfide electrolyte with ultrahigh moisture stability and ionic conductivity. Adv. Mater. 2021, 33, 2100921. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, L.; Zhou, D.; Weng, W.; Yao, X. Flexible sulfide electrolyte thin membrane with ultrahigh ionic conductivity for all-solid-state lithium batteries. Nano Lett. 2021, 21, 5233–5239. [Google Scholar] [CrossRef]
- Jiang, T.; He, P.; Wang, G.; Shen, Y.; Nan, C.W.; Fan, L.Z. Solvent-free synthesis of thin, flexible, nonflammable garnet-based composite solid electrolyte for all-solid-state lithium batteries. Adv. Energy Mater. 2020, 10, 1903376. [Google Scholar] [CrossRef]
- Thieme, S.; Brückner, J.; Bauer, I.; Oschatz, M.; Borchardt, L.; Althues, H.; Kaskel, S. High capacity micro-mesoporous carbon–sulfur nanocomposite cathodes with enhanced cycling stability prepared by a solvent-free procedure. J. Mater. Chem. A 2013, 1, 9225–9234. [Google Scholar] [CrossRef]
- Yao, W.; Chouchane, M.; Li, W.; Bai, S.; Liu, Z.; Li, L.; Sayahpour, B.; Shimizu, R.; Raghavendran, G.; Meng, Y.S. A 5 V-class cobalt-free battery cathode with high loading enabled by dry coating. Energy Environ. Sci. 2023, 16, 1620–1630. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, M.; Gao, H.; Hou, D.; Yu, C.; Liu, C.; Zhang, D.; Wu, J.C.; Yang, J.; Chen, D. Dense integration of solvent-free electrodes for Li-ion supercabattery with boosted low temperature performance. J. Power Sources 2020, 473, 228553. [Google Scholar] [CrossRef]
- Zhang, Y.; Huld, F.; Lu, S.; Jektvik, C.; Lou, F.; Yu, Z. Revisiting polytetrafluorethylene binder for solvent-free lithium-ion battery anode fabrication. Batteries 2022, 8, 57. [Google Scholar] [CrossRef]
- Giles, H.F., Jr.; Mount, E.M., III; Wagner, J.R., Jr. Extrusion: The Definitive Processing Guide and Handbook; William Andrew: Norwich, UK, 2004. [Google Scholar]
- Crawford, D.E.; Casaban, J. Recent developments in mechanochemical materials synthesis by extrusion. Adv. Mater. 2016, 28, 5747–5754. [Google Scholar] [CrossRef]
- Thiry, J.; Krier, F.; Evrard, B. A review of pharmaceutical extrusion: Critical process parameters and scaling-up. Int. J. Pharm. 2015, 479, 227–240. [Google Scholar] [CrossRef]
- Dreger, H.; Haselrieder, W.; Kwade, A. Influence of dispersing by extrusion and calendering on the performance of lithium-ion battery electrodes. J. Energy Storage 2019, 21, 231–240. [Google Scholar] [CrossRef]
- Gueguen, M.; Billion, M.; Majastre, H. Method of Manufacturing a Multilayer Electrochemical Assembly Comprising an Electrolyte between Two Electrodes, and an Assembly Made Thereby. U.S. Patent US5593462A, 10 September 1997. [Google Scholar]
- Jardiel, T.; Sotomayor, M.E.; Levenfeld, B.; Várez, A. Optimization of the Processing of 8-YSZ Powder by Powder Injection Molding for SOFC Electrolytes. Int. J. Appl. Ceram. Technol. 2008, 5, 574–581. [Google Scholar] [CrossRef]
- Verdier, N.; Foran, G.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. Challenges in solvent-free methods for manufacturing electrodes and electrolytes for lithium-based batteries. Polymers 2021, 13, 323. [Google Scholar] [CrossRef]
- Sotomayor, M.E.; de La Torre-Gamarra, C.; Bucheli, W.; Amarilla, J.M.; Varez, A.; Levenfeld, B.; Sanchez, J.Y. Additive-free Li4Ti5O12 thick electrodes for Li-ion batteries with high electrochemical performance. J. Mater. Chem. A 2018, 6, 5952–5961. [Google Scholar] [CrossRef]
- Sotomayor, M.E.; de La Torre-Gamarra, C.; Levenfeld, B.; Sanchez, J.Y.; Varez, A.; Kim, G.T.; Passerini, S. Ultra-thick battery electrodes for high gravimetric and volumetric energy density Li-ion batteries. J. Power Sources 2019, 437, 226923. [Google Scholar] [CrossRef]
- de La Torre-Gamarra, C.; Sotomayor, M.E.; Sanchez, J.Y.; Levenfeld, B.; Várez, A.; Laïk, B.; Pereira-Ramos, J.P. High mass loading additive-free LiFePO4 cathodes with 500 μm thickness for high areal capacity Li-ion batteries. J. Power Sources 2020, 458, 228033. [Google Scholar] [CrossRef]
- Voillequin, B.; Ayme-Perrot, D.; Dufour, B.; Sonntag, P. Anode for a Cell of a Lithium-Ion Battery, Its Manufacturing Process and the Battery Incorporating It. U.S. Patent 9,112,200, 18 August 2015. [Google Scholar]
- Voillequin, B.; Ayme-Perrot, D.; Dufour, B.; Sonntag, P. Cathode for a Cell of a Lithium-Ion Battery, Its Manufacturing Process and the Battery Incorporating it. U.S. Patent 9,484,571, 1 November 2016. [Google Scholar]
- El Khakani, S.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Rochefort, D.; Dollé, M. Melt-processed electrode for lithium-ion battery. J. Power Sources 2020, 454, 227884. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Wang, Z.; Volkov, V.; Lou, F.; Yu, Z. Recent technology development in solvent-free electrode fabrication for lithium-ion batteries. Renew. Sustain. Energy Rev. 2023, 183, 113515. [Google Scholar] [CrossRef]
- Hyvärinen, M.; Jabeen, R.; Kärki, T. The modelling of extrusion processes for polymers—A review. Polymers 2020, 12, 1306. [Google Scholar] [CrossRef]
- Astafyeva, K.; Dousset, C.; Bureau, Y.; Stalmach, S.L.; Dufour, B. High Energy Li-Ion Electrodes Prepared via a Solventless Melt Process. Batter. Supercaps 2020, 3, 341–343. [Google Scholar] [CrossRef]














| Polymer Materials | Abbreviation | Molecular Formula | Tg (°C) | Tm (°C) | Tensile Strength (MPa) | Separator Properties | Process |
|---|---|---|---|---|---|---|---|
| Polyolefin: Polyethylene Polypropylene Tri-layer | PE PP PP/PE/PP | (C2H4)n (C3H6)n | −100 −25 [293] | 110 170 [293] | 12–15 31–41 [294] | -Robust chemical stability; -Excellent electrochemical insulation capabilities; -Exceptional mechanical proprieties [295,296,297,298]. | Dry and wet processes |
| Polyvinylidene fluoride | PVDF | (C2H2F2)n | −35 [299] | 171 [299] | 45–55 | -High temperature resistance; -High crystallinity; -Mechanical strength; -Electrical insulation and dielectric properties [300,301,302]. | Electrospinning |
| Polyvinyl alcohol | PVA | (C2H4O)n | 85 [303] | 230 [303] | -Good wettability; -A network pore structure [304]. | Electrospinning | |
| Polytetrafluoroethylene | PTFE | (C2F4)n | 110 to 130 [305] | 330 [305] | 10 | -High thermal stability;-Good mechanical strength; -Low electrical conductivity [306]. | Electrospinning |
| Polyurethane | PU | C27H36N2O10 | formulation | formulation | - | -Help mitigate interfacial degradation; -Enhanced battery performance [307]. | Electrospinning |
| Cellulose | - | (C6H10O5)n | - | - | - | -Strong electrolyte affinity; -Thermal stability; -Good wettability [308]. | Electrospinning |
| Polyethylene terephthalate | PET | (C10H8O4)n | - | - | - | -Thermal stability; -High tensile strength; -Stable electrochemical performance [309,310]. | Electrospinning |
| Dry Process Separator Based on PP | Wet Process Separator Based on PE |
|---|---|
| Suitable for high-power output in EVs | Potential for EV battery use |
| Lower manufacturing costs | It involves more steps and materials (solvents), leading to higher manufacturing costs. |
| Higher melting temperature (beneficial for higher temperature scenarios): 170 °C | Lower melting temperature: 110–135 °C |
| High tensile strength: 31–41 MPa | Tensile strength: 12–15 MPa |
| Dielectric constant: 1 MHz 2.2–2.6 | Dielectric constant: 1 MHz 2.3 |
| Ref. | Process | Electrode | Materials Wt. % | Thickness (µm) | Porosity (%) | Area Capacity (AC) (mAh/cm2), Mass Loading (ML) (mg/cm2) | Discharge Capacity (mAh/g) | Capacity Retention (%) |
|---|---|---|---|---|---|---|---|---|
| [22] | Wet | Cathode | NMC111:PVDF:CB 90:5:5 | - | 30 | - | 138 | 84%, after undergoing 50 rounds of charging and discharging at a 0.5 C rate within the voltage range of 2.8–4.3 V |
| Dry | NMC111:PVDF:CB 90:5:5 | 40–130 | 30 | - | 138 | 87%, after 50 cycles at 0.5 C between 2.8 and 4.3 V | ||
| Wet | LCO:PVDF:CB 90:5:5 | - | 30 | - | 115 | 58%, after undergoing 50 rounds of charging and discharging at a 0.5 C rate within the voltage range of 2.5–4.2 V | ||
| Dry | LCO:PVDF:CB 90:5:5 | 40–130 | 30 | - | 114 | 70%, after 50 cycles at 0.5 C between 2.5 and 4.2 V | ||
| [319] | Wet | NMC111:PVD:CB 19:1:1 | 32.6 | 41 | 7.65 mg/cm2 (ML) | 156 | 60%, after 300 cycles between 3.0 V and 4.3 V at 0.5 C | |
| 52 | 35 | 14.27 mg/cm2 (ML) | 157 | 65%, after 300 cycles between 3.0 V and 4.3 V at 0.5 C | ||||
| Dry | NMC111:PVDF:CB 19:1:1 wt. | 40.5 | 31 | 10.07 mg/cm2 (ML) | 155 | 80%, after 300 cycles between 3.0 V and 4.3 V at 0.5 C | ||
| [23] | Wet | NMC11:PVDF:CB 90:5:5 | 55 | 29–30 | 2.45m Ah/cm2 (AC) | 145 | 65%, after 500 cycles at 0.5 C. | |
| Dry | NMC111:PVDF:CB90:5:5 | 55 100 150 200 | 29–30 | 2.45 mAh/cm2 (AC) 5.80 mAh/cm2 (AC) 6.52 mAh/cm2 (AC) 9.11 mAh/cm2 (AC) | 150 <20 <20 <20 At 3 C. | 80%, after 500 cycles at 0.5 C. | ||
| [29] | Wet | Anode | Graphite:PVDF:CB 85:10:5 | - | - | PVDF 3 mAh/cm2 (AC) | - | - |
| Graphite:(FEP or TVH):CB 86:7:7 | ||||||||
| Dry | Graphite:PVDF:CB 85:10:5 | - | - | FEP 2.7 mAh/cm2 (AC) | 370 | 99%, after 50 cycles at 0.5 C | ||
| Graphite:(FEP or TVH):CB 86:7:7 | TVH 3.5m Ah/cm2 (AC) | 345 | 97%, after 50 cycles at 0.5 C |
| Parameter | Dry-Painted Electrodes | Conventional Wet Electrodes | Implications |
|---|---|---|---|
| Cyclic voltammetry (CV) at 0.025 mV/s | Single pair of oxidation and reduction peaks (reduction at ~3.8 V, oxidation at ~4 V) | Single pair of oxidation and reduction peaks (reduction at ~3.8 V, oxidation at ~4 V) | Both types of electrodes show good reversibility of lithium insertion and extraction from LCO |
| CV peak symmetry at higher scan rates | Maintain symmetrical shape of cathodic and anodic peaks | Significant change in the shape of cathodic and anodic peaks | Dry-painted electrodes exhibit better rate capabilities and stability under rapid charge/discharge conditions |
| Potential difference between peaks | Smaller potential difference between cathodic and anodic peaks | The larger potential difference between cathodic and anodic peaks | Dry-painted electrodes have lower electrochemical polarization, indicating more efficient electrochemical reactions |
| EIS analysis | Show lower impedance characteristics | Show higher impedance characteristics | Lower impedance in dry-painted electrodes suggests better conductivity and lower internal resistance |
| Charge transfer resistance | Lower | Higher | Lower charge transfer resistance in dry-painted electrodes implies more efficient electron transfer during electrochemical reactions |
| Lithium-ion diffusion | Potentially more favorable | Potentially less favorable | Better lithium-ion diffusion in painted electrodes could contribute to their enhanced performance |
| Criteria | With Solvent [89,365] | Without Solvent [332,358,364] |
|---|---|---|
| Manufacturing process | Involves slurry-casting procedure | Utilizes melt extrusion technique |
| Active materials | Limited option | Diverse (LFP, NMC, LTO, NCA, …) |
| Binders | Conventional binders | PPC, HNRP,… |
| Thickness | Varies | Up to 500 µm |
| Density | - | ~2.9 g/cm3 |
| Volumetric capacity | - | ~349 mAh/cm3 |
| Areal capacity | - | >15.2 mAh/cm2 |
| Porosity | Uncontrolled | Controlled |
| Electrochemical performance | Varies | Enhanced |
| Dry Method | Process Description | Active Materials | Binder and Proprieties | Temperature | Chief Performance | References | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|
| Dry spray deposition | Utilizes a spraying gun | NMC, LCO | PVDF Thermoplastic | 100–190 °C 250 °C | Increased bonding strength | [22,24] |
|
|
| NMC | PVDF Thermoplastic | 170 °C | 80%: highest capacity retention after 300 cycles | [319] | ||||
| NMC | PVDF Thermoplastic | 180 °C | (>650 cycles) at a standard thickness of 56 µm. Electrodes (up to 200 µm) | [23] | ||||
| NMC | PVDF Thermoplastic | 200 °C | High molecular weight: PVDF is preferred | [329] | ||||
| Graphite | FEP/THV | 170–300 °C | New binders. High electrochemical performance | [29] | ||||
| Polymer fibrillation | Utilization of fibrillizable PTFE | LFP, LTO + 40% AC. | PTFE | 160–180 °C | Higher capacity and energy density | [350] |
|
|
| NMC, graphite Silicon/graphite | PTFE | 120 °C | High loading: 5 mAh/cm2, thick electrode, high-rate capability | [28] | ||||
| Graphite, hard carbon, soft carbon | PTFE | 160–180 °C | Excellent cycle life and high stability of hard and soft carbon | [351] | ||||
| NMC, CNF | PTFE | 320–330 °C | 100 charge–discharge cycles without artificial pressure, high areal 6.5 mAh/cm2. | [31] | ||||
| Extrusion and melt process | Extruded to form a continuous, uniform electrode film | LFP | PP PW SA Thermoplastic | 160–190 °C | Cathode exhibits good cyclability across 20 cycles at a C/10 rate—high areal capacity (13.7 mAh/cm−2) with 500 µm thickness. | [361] |
|
|
| LFP, NMC LTO | PPC elastomeric HNBR | 40–90 °C | A new binder, such as HNBR, decreases the viscosity, control of thickness, and porosity. | [364] | ||||
| NCA, graphite | PPC elastomeric | 50–250 °C | Areal capacities over 5 mAh/cm2 at a C/5 rate, large loading range from 4–40 mg/cm2. | [367] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouguern, M.D.; Madikere Raghunatha Reddy, A.K.; Li, X.; Deng, S.; Laryea, H.; Zaghib, K. Engineering Dry Electrode Manufacturing for Sustainable Lithium-Ion Batteries. Batteries 2024, 10, 39. https://doi.org/10.3390/batteries10010039
Bouguern MD, Madikere Raghunatha Reddy AK, Li X, Deng S, Laryea H, Zaghib K. Engineering Dry Electrode Manufacturing for Sustainable Lithium-Ion Batteries. Batteries. 2024; 10(1):39. https://doi.org/10.3390/batteries10010039
Chicago/Turabian StyleBouguern, Mohamed Djihad, Anil Kumar Madikere Raghunatha Reddy, Xia Li, Sixu Deng, Harriet Laryea, and Karim Zaghib. 2024. "Engineering Dry Electrode Manufacturing for Sustainable Lithium-Ion Batteries" Batteries 10, no. 1: 39. https://doi.org/10.3390/batteries10010039