An Industrial Perspective and Intellectual Property Landscape on Solid-State Battery Technology with a Focus on Solid-State Electrolyte Chemistries
Abstract
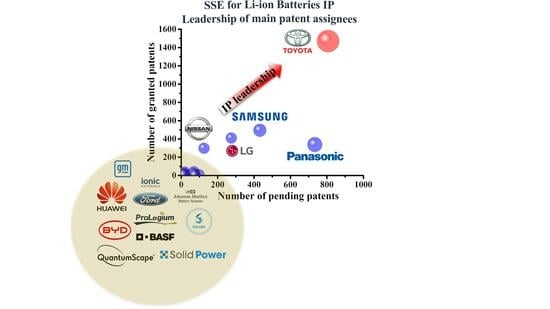
:1. Introduction
2. Facts and Discussion
2.1. Solid Polymer Electrolytes
2.2. Oxide-Based Solid Electrolyte
2.2.1. Garnet
2.2.2. Perovskite
2.2.3. NASICON-Type Oxides
2.3. Interphase Layer and Hybrid Electrolyte
2.4. Sulfide-Based Solid Electrolyte
3. Outlook
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical Challenges and Future Perspectives of All-Solid-State Lithium-Metal Batteries. Chem 2019, 5, 753–785. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Yim, C.-H.; Karkar, Z.; Abu-Lebdeh, Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]
- Yu, J.; Liu, J.; Lin, X.; Law, H.M.; Zhou, G.; Kwok, S.C.; Robson, M.J.; Wu, J.; Ciucci, F. A solid-like dual-salt polymer electrolyte for Li-metal batteries capable of stable operation over an extended temperature range. Energy Storage Mater. 2021, 37, 609–618. [Google Scholar] [CrossRef]
- Schmaltz, T.; Hartmann, F.; Wicke, T.; Weymann, L.; Neef, C.; Janek, J. A Roadmap for Solid-State Batteries. Adv. Energy Mater. 2023, 13, 2301886. [Google Scholar] [CrossRef]
- Woolley, H.M.; Vargas-Barbosa, N.M. Hybrid solid electrolyte-liquid electrolyte systems for (almost) solid-state batteries: Why, how, and where to? J. Mater. Chem. A 2023, 11, 1083–1097. [Google Scholar] [CrossRef]
- Lingappan, N.; Lee, W.; Passerini, S.; Pecht, M. A comprehensive review of separator membranes in lithium-ion batteries. Renew. Sustain. Energy Rev. 2023, 187, 113726. [Google Scholar] [CrossRef]
- Volkswagen Sinks Another $200 Million into Solid-State Battery Company QuantumScape. Available online: https://tcrn.ch/3db8Owu (accessed on 17 October 2023).
- BMW Gets First Batch of Solid-State Batteries From Solid Power. Available online: https://www.bmwblog.com/2023/11/12/bmw-gets-solid-state-batteries-from-solid-power/ (accessed on 17 October 2023).
- Colorado’s Solid Power Ramps up Solid-State Battery Cell Production. Available online: https://www.denverpost.com/2023/09/03/solid-power-expands-battery-cell-production/ (accessed on 17 October 2023).
- Hyundai Teams up with Its Electric to Test Curbside EV Charging. Available online: https://electrek.co/2023/04/18/hyundai-itselectric-test-curbside-ev-charging/ (accessed on 17 October 2023).
- Horowitz, Y.; Schmidt, C.; Yoon, D.-H.; Riegger, L.M.; Katzenmeier, L.; Bosch, G.M.; Noked, M.; Ein-Eli, Y.; Janek, J.; Zeier, W.G.; et al. Between liquid and all solid: A prospect on electrolyte future in lithium-ion batteries for electric vehicles. Energy Technol. 2020, 8, 2000580. [Google Scholar] [CrossRef]
- The Canadian Critical Minerals Strategy, from Exploration to Recycling: Powering the Green and Digital Economy for Canada and the World, His Majesty the King in Right of Canada, as Represented by the Minister of Natural Resources; Natural Resources Canada: Ottawa, ON, Canada, 2022.
- Espacenet Database. Available online: https://worldwide.espacenet.com/ (accessed on 17 October 2023).
- Song, Z.; Chen, F.; Martinez-Ibañez, M.; Feng, W.; Forsyth, M.; Zhou, Z.; Armand, M.; Zhang, H. A reflection on polymer electrolytes for solid-state lithium metal batteries. Nat. Commun. 2023, 14, 4884. [Google Scholar] [CrossRef] [PubMed]
- Mauger, A.; Julien, C.M. Solid Polymer Electrolytes for Lithium Batteries: A Tribute to Michel Armand. Inorganics 2022, 10, 110. [Google Scholar] [CrossRef]
- Keller, M.; Varzi, A.; Passerini, S. Hybrid electrolytes for lithium metal batteries. J. Power Sources 2018, 392, 206–225. [Google Scholar] [CrossRef]
- Liang, J.; Chen, D.; Adair, K.; Sun, Q.; Holmes, N.G.; Zhao, Y.; Sun, Y.; Luo, J.; Li, R.; Zhang, L.; et al. Insight into Prolonged Cycling Life of 4 V All-Solid-State Polymer Batteries by a High-Voltage Stable Binder. Adv. Energy Mater. 2021, 11, 2002455. [Google Scholar] [CrossRef]
- Arya, A.; Sadiq, M.; Sharma, A.L. Structural, electrical and ion transport properties of free-standing blended solid polymeric thin films. Polym. Bull. 2019, 76, 5149–5172. [Google Scholar] [CrossRef]
- Hovington, P.; Lagacé, M.; Guerfi, A.; Bouchard, P.; Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. New lithium metal polymer solid state battery for an ultrahigh energy: Nano C-LiFePO4 versus nano Li1. 2V3O8. Nano Lett. 2015, 15, 2671–2678. [Google Scholar] [CrossRef]
- Perea, A.; Dontigny, M.; Zaghib, K. Safety of solid-state Li metal battery: Solid polymer versus liquid electrolyte. J. Power Sources 2017, 359, 182–185. [Google Scholar] [CrossRef]
- Scrosati, B. History of lithium batteries. J. Solid State Electrochem. 2011, 15, 1623–1630. [Google Scholar] [CrossRef]
- Available online: https://www.bluebus.fr/en/about-us (accessed on 17 October 2023).
- Meabe, L.; Aldalur, I.; Lindberg, S.; Arrese-Igor, M.; Armand, M.; Martinez-Ibañez, M.; Zhang, H. Solid-State Electrolytes for Safe Rechargeable Lithium Metal Batteries: A Strategic View. Mater. Futures 2023, 2, 3. [Google Scholar] [CrossRef]
- Stettner, T.; Lingua, G.; Falco, M.; Balducci, A.; Gerbaldi, C. Protic ionic liquids-based crosslinked polymer electrolytes: A new class of solid electrolytes for energy storage devices. Energy Technol. 2020, 8, 2000742. [Google Scholar] [CrossRef]
- Porcarelli, L.; Gerbaldi, C.; Bella, F.; Nair, J.R. Super soft all-ethylene oxide polymer electrolyte for safe all-solid lithium batteries. Sci. Rep. 2016, 6, 19892. [Google Scholar] [CrossRef] [PubMed]
- Appetecchi, G.B.; Henderson, W.; Villano, P.; Berrettoni, M.; Passerini, S. PEO-LiN (SO2 CF2 CF3) 2 Polymer Electrolytes: I. XRD, DSC, and Ionic Conductivity Characterization. J. Electrochem. Soc. 2001, 148, A1171. [Google Scholar] [CrossRef]
- Shan, C.; Wang, Y.; Liang, M.; Lu, K.; Xiong, C.; Hu, W.; Liu, B. A comprehensive review of single ion-conducting polymer electrolytes as a key component of lithium metal batteries: From structural design to applications. Energy Storage Mater. 2023, 63, 102955. [Google Scholar] [CrossRef]
- Meabe, L.; Goujon, N.; Li, C.; Armand, M.; Forsyth, M.; Mecerreyes, D. Single-Ion Conducting Poly (Ethylene Oxide Carbonate) as Solid Polymer Electrolyte for Lithium Batteries. Batter. Supercaps 2020, 3, 68–75. [Google Scholar] [CrossRef]
- Porcarelli, L.; Shaplov, A.S.; Salsamendi, M.; Nair, J.R.; Vygodskii, Y.S.; Mecerreyes, D.; Gerbaldi, C. Single-ion block copoly (ionic liquid) s as electrolytes for all-solid state lithium batteries. ACS Appl. Mater. Interfaces 2016, 8, 10350–10359. [Google Scholar] [CrossRef] [PubMed]
- Lozinskaya, E.I.; Ponkratov, D.O.; Malyshkina, I.A.; Grysan, P.; Lingua, G.; Gerbaldi, C.; Vygodskii, Y.S. Self-assembly of Li single-ion-conducting block copolymers for improved conductivity and viscoelastic properties. Electrochim. Acta 2022, 413, 140126. [Google Scholar] [CrossRef]
- Guzmán-González, G.; Vauthier, S.; Alvarez-Tirado, M.; Cotte, S.; Castro, L.; Guéguen, A.; Casado, N.; Mecerreyes, D. Single-Ion Lithium Conducting Polymers with High Ionic Conductivity Based on Borate Pendant Groups. Angew. Chem. Int. Ed. Engl. 2021, 61, e202114024. [Google Scholar] [CrossRef]
- Alvarez-Tirado, M.; Guzmán-González, G.; Vauthier, S.; Cotte, S.; Guéguen, A.; Castro, L.; Mecerreyes, D. Designing Boron-Based Single-Ion Gel Polymer Electrolytes for Lithium Batteries by Photopolymerization. Macromol. Chem. Phys. 2022, 223, 2100407. [Google Scholar] [CrossRef]
- Lozinskaya, E.I.; Ponkratov, D.O.; Shaplov, A.S.; Malyshkina, I.A.; Streltsov, D.R.; Bakirov, A.V. Methacrylate Single-Ion Conducting Block Copolymers: Effect of the Chemical Structure on Conductivity and Morphological Organization. Polym. Sci. Ser. A 2023, 65, 36–52. [Google Scholar] [CrossRef]
- Porcarelli, L.; Aboudzadeh, M.A.; Rubatat, L.; Nair, J.R.; Shaplov, A.S.; Gerbaldi, C.; Mecerreyes, D. Single-ion triblock copolymer electrolytes based on poly(ethylene oxide) and methacrylic sulfonamide blocks for lithium metal batteries. J. Power Sources 2017, 364, 191–199. [Google Scholar] [CrossRef]
- Lingua, G.; Grysan, P.; Vlasov, P.S.; Verge, P.; Shaplov, A.S.; Gerbaldi, C. Unique carbonate-based single ion conducting block copolymers enabling high-voltage, all-solid-state lithium metal batteries. Macromolecules 2021, 54, 6911–6924. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Z.; Yu, L.; Zhang, P.; Wei, H.; Yu, Y. A new soft-matter material with old chemistry: Passerini multicomponent polymerization-induced assembly of AIE-active double-helical polymers with rapid visible-light degradability. Chem. Sci. 2020, 11, 8224–8230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.; Wang, C.; Cao, L.; Wang, Q.; Sun, J. L-glutamic acid as a versatile platform for rapid synthesis of functional polyesters via facile Passerini multicomponent polymerization. J. Polym. Sci. 2021, 59, 3111–3121. [Google Scholar] [CrossRef]
- Wan, J.; Liu, X.; Diemant, T.; Wan, M.; Passerini, S.; Paillard, E. Single-ion Conducting Interlayers for Improved Lithium Metal Plating. Energy Storage Mater. 2023, 63, 103029. [Google Scholar] [CrossRef]
- Seki, S. Solvent-Free 4 V-Class All-Solid-State Lithium-Ion Polymer Secondary Batteries. ChemistrySelect 2017, 2, 3848–3853. [Google Scholar] [CrossRef]
- Seki, S.; Kobayashi, Y.; Miyashiro, H.; Mita, Y.; Iwahori, T. Fabrication of high-voltage, high-capacity all-solid-state lithium polymer secondary batteries by application of the polymer electrolyte/inorganic electrolyte composite concept. Chem. Mater. 2005, 17, 2041–2045. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Chen, B.; Wang, L.; Yue, L.; Liu, H.; Cui, G. A strategy to make high voltage LiCoO2 compatible with polyethylene oxide electrolyte in all-solid-state lithium ion batteries. J. Electrochem. Soc. 2017, 164, A3454. [Google Scholar] [CrossRef]
- Ahniyaz, A.; de Meatza, I.; Kvasha, A.; Garcia-Calvo, O.; Ahmed, I.; Sgroi, M.F.; Zhang, N. Progress in solid-state high voltage lithium-ion battery electrolytes. Adv. Appl. Energy 2021, 4, 100070. [Google Scholar] [CrossRef]
- Werwitzke, C. Actually, we are the pioneer of solid-state battery. Electrive. Com. 2021. Available online: https://www.electrive.com/2021/03/03/actually-we-are-the-pioneer-of-solid-state-battery/ (accessed on 17 October 2023).
- Monconduit, L.; Croguennec, L. (Eds.) Prospects for Li-Ion Batteries and Emerging Energy Electrochemical Systems; World Scientific: Location, UK, 2018; Volume 4. [Google Scholar]
- Lecuyer, M.; Szymczak, J.; Deschamps, M. Membrane Séparatrice Hybride Pour Batterie. FR3112244A1, 6 July 2020. [Google Scholar]
- Ferrand, A.; Gigmes, D.; Lassagne, A.; Trang, P.; Pavlenko, E.; Bouchet, R.; Lecuyer, M.; Deschamps, M. Polymer Electrolyte for a Lithium Metal Polymer Battery Having Improved Performance. FR3088932A1, 27 November 2018. [Google Scholar]
- Bouchet, R.; Deschamps, M. Batterie Lithium Métal Polymère à Haute Densité D’énergie. FR3049114A1, 18 March 2016. [Google Scholar]
- Deschamps, M.; Lecuyer, M.; Bouchet, R.; Roland, J. Utilisation D’un Mélange de Sels à Titre D’additif Dans Une Batterie au Lithium Gélifiée. FR3073982A1, 20 November 2017. [Google Scholar]
- Deschamps, M.; Lecuyer, M. Use of Lithium Nitrate as the Sole Lithium Salt in a Lithium Gel Battery. FR3073984A1, 20 November 2017. [Google Scholar]
- Bernardo, P.; Bodenez, V.; Deschamps, M.; Dru, M.; Lecuyer, M. Electrolytes Polymères Solides Comprenant Des Polymères Solubles, Des Sels de Lithium et Des Polymères Halogénés Sélectionnés, et Batteries Les Comprenant. FR3071360A1, 18 September 2017. [Google Scholar]
- Lecuyer, M.; Bodenez, V.; Bernardo, P.; Dru, M.; Deschamps, M. Procédé de Traitement Thermique D’une Batterie au Lithium. FR3052599A1, 8 June 2016. [Google Scholar]
- Available online: https://www.lifepo4ag.com/hydro-quebec (accessed on 17 October 2023).
- Daigle, J.; Larouche, A.; Rochon, S.; Krachkovskiy, S.; Barray, F.; Hamel-Paquet, J. Conductive Sulfamide Polymers, Use Therof in Batteries and Method for Producing Same. WO2022126251A1, 23 June 2022. [Google Scholar]
- Daigle, J.-C.; Larouche, A.-P.; Charest, P.; Guerfi, A.; Dontigny, M.; Armand, M.; Zaghib, K. Polymers Based on Ionic Monomers, Compositions Comprising Same, Methods for Manufacturing Same, and Use Thereof in Electrochemical Applications. WO2021146815A1, 29 July 2021. [Google Scholar]
- Zaghib, K.; Perrier, M.; Guerfi, A.; Dupuis, E.; Charest, P.; Allaire, F.; Armand, M. Polymer Electrolyte with High Stability, Its Use in Electrochemical Systems. US7897674B2, 1 March 2011. [Google Scholar]
- Harvey, P.; Sanchez, J.; Alloin, F. Copolymer of Ethylene Oxide and at Least One Substituted Oxirane Carrying a Cross-Linkable Function, Process for Preparation Thereof and Use Thereof for Producing Materials with Ionic Conduction. US6903174B2, 7 June 2005. [Google Scholar]
- Paolella, A.; Gagnon, C.; Perea, A.; Guerfi, A.; Zaghib, K. Protective Material for a Lithium Metal Anode: Method for Its Preparation and Use. WO2020206531A1, 15 October 2020. [Google Scholar]
- Daigle, J.-C.; Barray, F.; Guerfi, A.; Fleutot, B.; Garitte, E.; Krachkovskiy, S.; Koh, K. Ionic plastic crystals, compositions comprising same, methods for manufacturing same and uses thereof. WO2022165598A1, 11 August 2022. [Google Scholar]
- Paolella, A.; Savoie, S.; Girard, G.; Forand, A.; Zhu, W.; Guerfi, A.; Zaghib, K. Multilayer Electrode-Eletrolyte Components and Their Production Methods. CA3135270A1, 12 November 2020. [Google Scholar]
- Available online: https://www.brightvolt.com/our-technology/ (accessed on 17 October 2023).
- Anani, A. Solid Polymer Matric Electrolyte (PME) for Rechargeable Lithium Batteries and Batteries Made Therewith. WO2020033538A1, 13 February 2020. [Google Scholar]
- Gustafson, S.D.; Antonucci, J.T. Polyimide Battery. US5888672A, 30 March 1999. [Google Scholar]
- Wensley, C.G.; Gustafson, S.; Singleton, R.W.; Vallee, A.; Brouillette, D. Polyimide Matrix Electrolyte and Improved Batteries Therefrom. US7129005B2, 31 October 2006. [Google Scholar]
- Wensley, C.G.; Vallee, A.; Brouillette, D.; Gustafson, S. Polyimide Matrix Electrolyte. US7198870B2, 3 April 2007. [Google Scholar]
- Nelson, C.; Wensley, G.; Guindy, W. Lithium Inks and Electrodes and Batteries Made Therefrom. US2013011735A1, 10 January 2013. [Google Scholar]
- Kreitman, N. Batteries Comprising a Multilayer Dielectric and Sealing Film and Method of Making the Batteries. US10991921B2, 27 April 2021. [Google Scholar]
- Lux Research Website. Available online: https://www.luxresearchinc.com/ (accessed on 17 October 2023).
- Li, S.; Taggougui, M.; Leitner, A.; Al-Obeidi, A. Polymer Electrolytes with Improved Ionic Conductivity. WO2023201016A1, 19 October 2023. [Google Scholar]
- Bonnick, P.; Redko, M.; Wang, C.; Frey, M.; Jones, M.; Wang, S.; Muldoon, J. An Exploration of the Role of Charge-Transfer Complexes in Polymer Electrolytes. ACS Energy Lett. 2023, 8, 4251–4258. [Google Scholar] [CrossRef]
- Al-Salih, H.; Houache MS, E.; Baranova, E.A.; Abu-Lebdeh, Y. Composite Cathodes for Solid-State Lithium Batteries: “Catholytes” the Underrated Giants. Adv. Energy Sustain. Res. 2022, 3, 2200032. [Google Scholar] [CrossRef]
- Zimmerman, M.; Leising, R. Battery Electrode with Solid Polymer Electrolyte and Aqueous Soluble Binder. US2021119213A1, 22 April 2021. [Google Scholar]
- Zimmerman, M. Solid Electrolyte High Energy Battery. US9819053B1, 14 November 2017. [Google Scholar]
- Zimmerman, M. Solid Ionically Conducting Polymer Material. US20170018781A1, 19 January 2017. [Google Scholar]
- Ryu, J.; Guilong, J.; Ahn, J.; Kang, S.; Lee, J. Hybrid Solid Electrolyte Membrane for All Solid State Secondary Battery and All Solid State Secondary Battery Comprising the Same. KR20190113605A, 8 October 2019. [Google Scholar]
- Lee, J.; Jung, H.; Cho, S.; Kim, S. Solid-State Battery Anode Comprising Polymer Layer for Preventing Micro Short-Circuit and Solid-State Battery Comprising Same. WO2022065813A1, 31 March 2022. [Google Scholar]
- Kim, D.; Chae, J.; Kim, S.; Lim, S.; Ahn, J. Solid Electrolyte, Method for Preparing Same, and All-Solid-State Battery Including Same. US2021028439A1, 28 January 2021. [Google Scholar]
- Ahn, K.; Kim, D.; Lee, W.; Jeong, B.; Lim, D.; Lee, C.; Jeong, Y. Gel Polymer Electrolyte and Lithium Secondary Battery Comprising Same. WO2023008970A1, 2 February 2023. [Google Scholar]
- Abouali, S.; Yim, C.H.; Merati, A.; Abu-Lebdeh, Y.; Thangadurai, V. Garnet-based solid-state Li batteries: From materials design to battery architecture. ACS Energy Lett. 2021, 6, 1920–1941. [Google Scholar] [CrossRef]
- Yan, S.; Yim, C.H.; Pankov, V.; Bauer, M.; Baranova, E.; Weck, A.; Abu-Lebdeh, Y. Perovskite Solid-State Electrolytes for Lithium Metal Batteries. Batteries 2021, 7, 75. [Google Scholar] [CrossRef]
- Hou, M.; Liang, F.; Chen, K.; Dai, Y.; Xue, D. Challenges and perspectives of NASICON-type solid electrolytes for all-solid-state lithium batteries. Nanotechnology 2020, 31, 132003. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guo, C.; Naveed, A.; Lei, J.; Yang, J.; Nuli, Y.; Wang, J. Recent progress and perspective on lithium metal anode protection. Energy Storage Mater. 2018, 14, 199–221. [Google Scholar] [CrossRef]
- Sakka, Y.; Yamashige, H.; Watanabe, A.; Takeuchi, A.; Uesugi, M.; Uesugi, K.; Orikasa, Y. Pressure dependence on the three-dimensional structure of a composite electrode in an all-solid-state battery. J. Mater. Chem. A 2022, 10, 16602–16609. [Google Scholar] [CrossRef]
- Gonzalez Puente, P.M.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Chen, F. Garnet-type solid electrolyte: Advances of ionic transport performance and its application in all-solid-state batteries. J. Adv. Ceram. 2021, 10, 933–972. [Google Scholar] [CrossRef]
- Thompson, T.; Wolfenstine, J.; Allen, J.L.; Johannes, M.; Huq, A.; David, I.N.; Sakamoto, J. Tetragonal vs. cubic phase stability in Al–free Ta doped Li7La3Zr2O12 (LLZO). J. Mater. Chem. A 2014, 2, 13431–13436. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, F.; Yang, W.; Yang, J.; Ma, X.; Chen, D.; Zhang, L. Dual regulation of Li+ migration of Li6. 4La3Zr1. 4M0. 6O12 (M = Sb, Ta, Nb) by bottleneck size and bond length of M–O. J. Am. Ceram. Soc. 2020, 103, 2483–2490. [Google Scholar] [CrossRef]
- Hyeojlo, G.; Sungkyun, J.; Jusik, K. Solid Ion Conductor, Solid Electrolyte Including the Same, Electrochemical Device Including the Same, and Preparation Method Thereof. US20220044837A1, 10 February 2022. [Google Scholar]
- Kim, J.; Lee, M.; Kim, S.; Badding, M.E.; Chang, J.; Song, Z. All-Solid Secondary Battery and Method of Manufacturing the Same. US2022/0045354A1, 10 February 2022. [Google Scholar]
- Chang, W.; Hood, Z.; Rupp, J.; Miara, L. Bilayer Component for a Lithium Battery. US20210305621A1, 30 September 2021. [Google Scholar]
- Johnson Matthey Website. Available online: https://matthey.com/ (accessed on 17 October 2023).
- Nuttall, C.; Petrucco, E. Lithium-Ion Conductive Ceramic Material and Process. WO2021/224637A1, 11 November 2021. [Google Scholar]
- Li, J.; Ma, Z.F. Past and present of LiFePO4: From fundamental research to industrial applications. Chem 2019, 5, 3–6. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, Z.; Li, B. Solid Electrolyte and Solid Lithium Battery. CN112436181A, 2 March 2021. [Google Scholar]
- Solvay Website. Available online: https://www.solvay.com/en (accessed on 17 October 2023).
- Bertry, L.; Toth, R.; Le Mercier, T.; Buissette, V.; Guerin, K.; Dubois, M.; Herraiz, M. Process for Fluorination of an LLZO Garnet. WO 2021219806A1, 4 November 2021. [Google Scholar]
- Choi, A.; Donnelly, N.; Iyer, S.; Singh, J.; Xu, G.; Friedland, J.; Sarma, H.; Shah, N. Sintering Large Area Ceramic Films. WO2020081718A1, 23 April 2020. [Google Scholar]
- Arnold, T.; Beck, L.; Ho, T.; Yang, A. Electrochemical Cells with Catholyte Additives and Lithium-Stuffed Garnet Separators. CN113039671A, 25 June 2021. [Google Scholar]
- Van Berkel, K.; Jeffries, P. High Green Density Ceramics for Batteries. CN115087629A, 20 September 2022. [Google Scholar]
- Li, Y.; Han, J.T.; Wang, C.A.; Xie, H.; Goodenough, J.B. Optimizing Li+ conductivity in a garnet framework. J. Mater. Chem. 2012, 22, 15357–15361. [Google Scholar] [CrossRef]
- Geng, H.; Lan, J.; Mei, A.; Lin, Y.; Nan, C.W. Effect of sintering temperature on microstructure and transport properties of Li3xLa2/3− xTiO3 with different lithium contents. Electrochim. Acta 2011, 56, 3406–3414. [Google Scholar] [CrossRef]
- Zheng, Z.; Fang, H.Z.; Liu, Z.K.; Wang, Y. A fundamental stability study for amorphous LiLaTiO3 solid electrolyte. J. Electrochem. Soc. 2014, 162, A244. [Google Scholar] [CrossRef]
- Available online: https://www.reuters.com/business/autos-transportation/toyota-market-next-gen-battery-evs-2026-built-by-new-ev-unit-2023-06-13/ (accessed on 17 October 2023).
- Ota, S.; Watanabe, M.; Osaki, M. Perovskite Ion-Conducting Oxide, Composite, and Lithum Secondary Battery. JP2020136123A, 31 August 2020. [Google Scholar]
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G.Y. Ionic conductivity of solid electrolytes based on lithium titanium phosphate. J. Electrochem. Soc. 1990, 137, 1023. [Google Scholar] [CrossRef]
- DeWees, R.; Wang, H. Synthesis and properties of NaSICON-type LATP and LAGP solid electrolytes. ChemSusChem 2019, 12, 3713–3725. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Nolan, A.M.; Lu, J.; Wang, J.; Yu, X.; Mo, Y.; Li, H. The thermal stability of lithium solid electrolytes with metallic lithium. Joule 2020, 4, 812–821. [Google Scholar] [CrossRef]
- Fan, X.; Zhong, C.; Liu, J.; Ding, J.; Deng, Y.; Han, X.; Zhang, J. Opportunities of flexible and portable electrochemical devices for energy storage: Expanding the spotlight onto semi-solid/solid electrolytes. Chem. Rev. 2022, 122, 17155–17239. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Belov, D. Contact Surface Adjusting Material for Solid Electrolytes and Composite Electrolyte System Thereof. US20210119245A1, 22 April 2021. [Google Scholar]
- Prologium Holding Inc. Solid Electrolyte Structure. DE 202022103923 U1, 23 June 2022.
- Kim, T.; Yoon, D.; Lim, H.; Jung, M.; Baek, J. Sulfide-Based Solid Electrolyte for a Secondary Batteries and Preparation Method Thereof. KR102560211B1, 24 July 2023. [Google Scholar]
- Kim, T.-H.; Lim, H.-S.; Jung, M.; Yoon, D. Solid Electrolyte Composition for Low Temperature Sintering and Solid Electrolyte Produced Using the Composition. KR102550168B1, 15 June 2016. [Google Scholar]
- Seon-min, W.; Kim, T.-H. Energy Storage System Including Sodium-Ion Batteries and Method for Operating the Same. KR101761031B1, 18 July 2017. [Google Scholar]
- Kim, T.; Lim, H.; Jung, M.; Yoon, D. Solid Electrolyte Composition for Low Temperature Sintering and Solid Electrolyte Produced Using the Composition. KR20220117398A, 24 August 2022. [Google Scholar]
- Al-Salih, H.; Cui, M.; Yim, C.H.; Sadighi, Z.; Yan, S.; Karkar, Z.; Goward Gillian, R.; Baranova Elena, A.; Abu-Lebdeh, Y. A Ceramic Rich Quaternary Composite Solid-State Electrolyte for Solid-State Lithium Metal Batteries. J. Electrochem. Soc. 2022, 169, 080510. [Google Scholar] [CrossRef]
- Al-Salih, H.; Huang, A.; Yim, C.H.; Freytag, A.I.; Goward, G.R.; Baranova, E.; Abu-Lebdeh, Y. A Polymer-Rich Quaternary Composite Solid Electrolyte for Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070557. [Google Scholar] [CrossRef]
- Ge, X.; Fu, C.; Chan, S.H. Double layer capacitance of anode/solid-electrolyte interfaces. Phys. Chem. Chem. Phys. 2011, 13, 15134–15142. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, S.; Li, Y.; Xin, S.; Manthiram, A.; Goodenough, J.B. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J. Am. Chem. Soc. 2016, 138, 9385–9388. [Google Scholar] [CrossRef]
- Lee, E.G.; Cho, B.G.; Han, S.I. Catholyte for Lithium Metal Battery Composite Cathode for Lithium Metal Battery Including the Same Preparing Method Thereof and Lithium Metal Battery Comprising the Same. KR20210112925A, 15 September 2021. [Google Scholar]
- Lee, M.; Kim, J.; Kim, S.; Roev, V.; Jung, C.; Han, I. All-Solid Secondary Battery and Method of Preparing the Same. US2022006125A1, 6 January 2022. [Google Scholar]
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 2018, 3, 16–21. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Hong, X. Composite Solid-State Electrolyte Material and Preparation Method Therefor, Lithium Secondary Battery, and Terminal. WO2021254220A1, 23 December 2021. [Google Scholar]
- Yang, D.; Xu, D.; Xie, F. Composite Separator, Electrochemical Device, and Electronic Apparatus. CN116053699A, 2 May 2023. [Google Scholar]
- Li, J.; Ma, Y.; Ren, J.; Guo, Z. Lithium ion Battery, Preparation Method Thereof and Electric Vehicle. CN110828883A, 21 February 2020. [Google Scholar]
- Kim, C.; Darwich, A.; Fleutot, B.; Garitte, E.; Koh, K.; Girard, M.; Gagnon, C.; Zaghib, K. Electrochemical Cells in the Solid State Methods for Preparing Same and Uses Thereof. WO2021237335A1, 1 December 2021. [Google Scholar]
- Zhang, X.; Daigle, J.; Kim, C. Composite Material Comprising a Fluorinated Amide and Uses Thereof in Electrochemical Cells. WO2022261785A1, 22 December 2022. [Google Scholar]
- Paolella, A.; Girard, G.; Forand, A.; Savoir, S.; La Monaca, A.; Guerfi, A.; Zaghib, K. Ceramic Electrolytes, Methods for Preparing Same and Electrochemical Cells Comprising Them. WO2020206552A1, 15 October 2020. [Google Scholar]
- Singh, M.; Hudson, W.; Yamago, S. Polyacrylonitrile Gels for Energy Storage. WO2019213159A1, 7 November 2019. [Google Scholar]
- Chen, Z.; Donelly, N.; Holme, T.; Singh, D. Solid Electrolyte Separator Bonding Agent. US11450926B2, 20 September 2022. [Google Scholar]
- Ren, D.; Lu, L.; Hua, R.; Zhu, G.; Liu, X.; Mao, Y.; Ouyang, M. Challenges and opportunities of practical sulfide-based all-solid-state batteries. Etransportation 2023, 18, 100272. [Google Scholar] [CrossRef]
- Gandi, S.; Vaddadi VS, C.S.; Panda SS, S.; Goona, N.K.; Parne, S.R.; Lakavat, M.; Bhaumik, A. Recent progress in the development of glass and glass-ceramic cathode/solid electrolyte materials for next-generation high capacity all-solid-state sodium-ion batteries: A review. J. Power Sources 2022, 521, 230930. [Google Scholar] [CrossRef]
- Morino, Y.; Tsukasaki, H.; Mori, S. Microscopic Degradation Mechanism of Argyrodite-Type Sulfide at the Solid Electrolyte–Cathode Interface. ACS Appl. Mater. Interfaces 2023, 15, 23051–23057. [Google Scholar] [CrossRef]
- Cai, Y.; Li, C.; Zhao, Z.; Mu, D.; Wu, B. Air Stability and Interfacial Compatibility of Sulfide Solid Electrolytes for Solid-State Lithium Batteries: Advances and Perspectives. ChemElectroChem 2022, 9, e202101479. [Google Scholar] [CrossRef]
- Tsukasaki, H.; Sano, H.; Igarashi, K.; Wakui, A.; Yaguchi, T.; Mori, S. Deterioration process of argyrodite solid electrolytes during exposure to humidity-controlled air. J. Power Sources 2022, 524, 231085. [Google Scholar] [CrossRef]
- Nikodimos, Y.; Huang, C.J.; Taklu, B.W.; Su, W.N.; Hwang, B.J. Chemical stability of sulfide solid-state electrolytes: Stability toward humid air and compatibility with solvents and binders. Energy Environ. Sci. 2022, 15, 991–1033. [Google Scholar] [CrossRef]
- Trevey, J.E.; Jung, Y.S.; Lee, S.H. Preparation of Li2S–GeSe2–P2S5 electrolytes by a single step ball milling for all-solid-state lithium secondary batteries. J. Power Sources 2010, 195, 4984–4989. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, Y.; Eom, M.; Shin, D. Characterization of amorphous and crystalline Li2S–P2S5–P2Se5 solid electrolytes for all-solid-state lithium ion batteries. Solid State Ion. 2012, 225, 626–630. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Lu, P.; Yan, W.; Yang, M.; Li, H.; Wu, F. Water-stable sulfide solid electrolyte membranes directly applicable in all-solid-state batteries enabled by superhydrophobic Li+-conducting protection layer. Adv. Energy Mater. 2022, 12, 2102348. [Google Scholar] [CrossRef]
- Tan, D.H.; Banerjee, A.; Deng, Z.; Wu, E.A.; Nguyen, H.; Doux, J.M.; Chen, Z. Enabling thin and flexible solid-state composite electrolytes by the scalable solution process. ACS Appl. Energy Mater. 2019, 2, 6542–6550. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Guo, W.; Li, M.; Qing, J.; Cai, C.; Yi, P.; Chen, W. Progress and Perspective of Glass-Ceramic Solid-State Electrolytes for Lithium Batteries. Materials 2023, 16, 2655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, S.H.; Zhang, X.; Guo, S.; Li, P.; Yan, S. Glass and glass ceramic electrodes and solid electrolyte materials for lithium ion batteries: A review. J. Non-Cryst. Solids 2023, 619, 122581. [Google Scholar] [CrossRef]
- Tao, B.; Zhong, D.; Li, H.; Wang, G.; Chang, H. Halide solid-state electrolytes for all-solid-state batteries: Structural design, synthesis, environmental stability, interface optimization and challenges. Chem. Sci. 2023, 14, 8693–8722. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cao, P. Solid-state inorganic electrolytes (oxides, sulfides, and halides). In Advanced Ceramics for Energy Storage, Thermoelectrics and Photonics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 77–117. [Google Scholar]
- Zhao, F.; Liang, J.; Yu, C.; Sun, Q.; Li, X.; Adair, K.; Sun, X. A versatile Sn-substituted argyrodite sulfide electrolyte for all-solid-state Li metal batteries. Adv. Energy Mater. 2020, 10, 1903422. [Google Scholar] [CrossRef]
- Liang, J.; Chen, N.; Li, X.; Li, X.; Adair, K.R.; Li, J.; Sun, X. Li10Ge (P1–x Sb x) 2S12 lithium-ion conductors with enhanced atmospheric stability. Chem. Mater. 2020, 32, 2664–2672. [Google Scholar] [CrossRef]
- Tsukasaki, H.; Morimoto, H.; Mori, S. Ionic conductivity and thermal stability of Li2O–Li2S–P2S5 oxysulfide glass. Solid State Ion. 2020, 347, 115267. [Google Scholar] [CrossRef]
- Ohtomo, T.; Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. Electrical and electrochemical properties of Li2S–P2S5–P2O5 glass–ceramic electrolytes. J. Power Sources 2005, 146, 715–718. [Google Scholar] [CrossRef]
- Jung, W.D.; Jeon, M.; Shin, S.S.; Kim, J.S.; Jung, H.G.; Kim, B.K.; Kim, H. Functionalized sulfide solid electrolyte with air-stable and chemical-resistant oxysulfide nanolayer for all-solid-state batteries. ACS Omega 2020, 5, 26015–26022. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fitzhugh, W.; Ye, L.; Ning, J.; Li, X. Advanced sulfide solid electrolyte by core-shell structural design. Nat. Commun. 2018, 9, 4037. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, S.; Lochala, J.; Desrochers, D.; Liu, B.; Zhang, W.; Xiao, J. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 2018, 11, 1803–1810. [Google Scholar] [CrossRef]
- Nakamura, T.; Amezawa, K.; Kulisch, J.; Zeier, W.G.; Janek, J. Guidelines for all-solid-state battery design and electrode buffer layers based on chemical potential profile calculation. ACS Appl. Mater. Interfaces 2019, 11, 19968–19976. [Google Scholar] [CrossRef]
- Nomoto, K.; Miyazaki, A. Solid State Battery. WO2021177212A1, 10 September 2021. [Google Scholar]
- Morita, K.; Yada, C.; Suzuki, Y. Method for Producing All Solid State State Battery. JP2022052690A, 4 April 2022. [Google Scholar]
- Sasaki, I.; Fujinoki, N.; Miyazaki, A.; Shiotani, S.; Sugiyama, T. Positive Electrode Material and Battery. WO2021157361A1, 12 August 2021. [Google Scholar]
- Sugiyama, T.; Shiotani, S.; Usami, T.; Miyazaki, A.; Sasaki, I. Positive Electrode Material and Battery. WO2022009806A1, 13 January 2022. [Google Scholar]
- Nagano, A. Method of Evaluating All-Solid-State Battery. JP2022076604A, 20 May 2022. [Google Scholar]
- Li, X. All Solid-State Battery and Manufacturing Method for All Solid-State Battery. EP4117058A1, 5 July 2022. [Google Scholar]
- Takeuchi, K.; Shiotani, S.; Otomo, T. Solid Electrolyte. JP2020061326A, 16 April 2020. [Google Scholar]
- Narimatsu, E.; Maeda, H.; Matsubara, K.; Kanno, R.; Hori, S. Solid Electrolyte for All Solid-State Battery and Production Method Therefor. WO2022231396A1, 3 November 2022. [Google Scholar]
- Lee, J.; Han, H.; Hah, H.; Kim, D. Manufacturing Method of All-Solid State Battery and All-Solid State Battery Thereby. KR20220140428A, 18 October 2022. [Google Scholar]
- Jung, H.; Lee, J.; Hah, H.; Han, H. All-Solid-State Battery. WO2022055189A1, 17 March 2022. [Google Scholar]
- Lee, J.; Choi, L. All-Solid-State Battery Comprising Two Types of Solid Electrolyte Layers. WO2022211447A1, 6 October 2022. [Google Scholar]
- BASF Website. Available online: https://www.basf.com/global/en/media/news-releases/2022/03/p-22-133.html (accessed on 17 October 2023).
- Adeli, P.; Zhizhen, Z.; Nazar, L.; Kulisch, J.; Wu, X. Lithium Ion Conducting Solid Materials. WO2021204922A1, 14 October 2021. [Google Scholar]
- Kaup, K.; Nazar, L.; Kulisch, J.; Wu, X. Lithium-Ion Conducting Haloboro-Oxysulfides. WO2020254314A1, 24 December 2020. [Google Scholar]
- Li, Z.; Kan, X.; Liu, H.; Su, Q.; Lu, Y. Solid State Battery Having Capacitor Auxiliary Interlayer. CN112242564A, 19 January 2021. [Google Scholar]
- Kim, S.; Kim, H.; Lee, S. Solid Electrolyte Electrochemical Cell Comprising Solid Electrolyte and Method for Preparing Solid Electrolyte. KR20210055581A, 17 May 2021. [Google Scholar]
- Suzuki, S.; Tsujimura, T.; Suzuki, N.; Yashiro, N. A Solid Electrolyte Material Solid Electrolyte Including the Same All Solid Secondary Battery Including the Solid Electrolyte and Method of Preparing the Solid Electrolyte Material. KR20220117106A, 23 August 2022. [Google Scholar]
- Wang, Y.; Miara, L.; Cho, J.; Jung, S.; Gwon, H. Solid-State Electrolyte, Solid-State Battery Including the Electrolyte, and Method of Making the Same. US2022037693A1, 3 February 2022. [Google Scholar]
- Becker, C.; Ruther, R.; Roberts, J.; Morrissey, I. Rheological Modified Slurries for Electrochemical Cells and Components Made Therefrom. WO2022174086A1, 18 August 2022. [Google Scholar]
- Carlson, B.; Culver, S.; Lisenker, I. Method of Synthesis of Solid Electrolyte, a Solid-State Electrolyte Composition, and an Electrochemical Cell. WO2022125961A1, 16 June 2022. [Google Scholar]
- Francisco, B.; Culver, S. Solid Electrolyte Material and Solid-State Battery Made Therewith. WO2022066924A2, 31 March 2022. [Google Scholar]
- Francisco, B.; Oberwetter, S. Solid-State Electrolyte Materials Having Increased Water Content. WO2023122124A1, 29 June 2023. [Google Scholar]
- Wan, J.; Xie, J.; Mackanic, D.G.; Burke, W.; Bao, Z.; Cui, Y. Status, promises, and challenges of nanocomposite solid-state electrolytes for safe and high performance lithium batteries. Mater. Today Nano 2018, 4, 1–16. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkar, Z.; Houache, M.S.E.; Yim, C.-H.; Abu-Lebdeh, Y. An Industrial Perspective and Intellectual Property Landscape on Solid-State Battery Technology with a Focus on Solid-State Electrolyte Chemistries. Batteries 2024, 10, 24. https://doi.org/10.3390/batteries10010024
Karkar Z, Houache MSE, Yim C-H, Abu-Lebdeh Y. An Industrial Perspective and Intellectual Property Landscape on Solid-State Battery Technology with a Focus on Solid-State Electrolyte Chemistries. Batteries. 2024; 10(1):24. https://doi.org/10.3390/batteries10010024
Chicago/Turabian StyleKarkar, Zouina, Mohamed S. E. Houache, Chae-Ho Yim, and Yaser Abu-Lebdeh. 2024. "An Industrial Perspective and Intellectual Property Landscape on Solid-State Battery Technology with a Focus on Solid-State Electrolyte Chemistries" Batteries 10, no. 1: 24. https://doi.org/10.3390/batteries10010024