From Micro to Nano: Grinding Natural Magnetite Ore for Microalgae Harvesting
Abstract
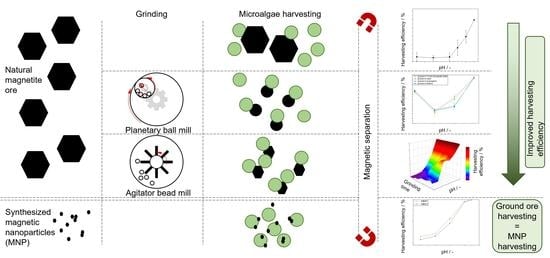
:1. Introduction
2. Materials and Methods
2.1. Microalgae
2.2. Magnetic Microparticles from Ore (MMO)
2.3. Magnetic Nanoparticles from Ore (MNO)—Preparation in a Planetary Ball Mill
2.4. Magnetic Nanoparticles from Ore (MNO)—Preparation in an Agitator Bead Mill
2.5. Magnetic Nanoparticle (MNP) Synthesis
2.6. Biomass Quantification
2.7. Harvesting Experiments
3. Results and Discussion
3.1. Preparation of and Harvesting with MNOs
3.1.1. Planetary Ball Mill
3.1.2. Agitator Bead Mill
3.2. Magnetic Micro- vs. Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Yang, S.; Xu, J.; Wang, Z.-M.; Bao, L.-J.; Zeng, E.Y. Cultivation of oleaginous microalgae for removal of nutrients and heavy metals from biogas digestates. J. Clean. Prod. 2017, 164, 793–803. [Google Scholar] [CrossRef]
- Hariskos, I.; Posten, C. Biorefinery of microalgae—opportunities and constraints for different production scenarios. Biotechnol. J. 2014, 9, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Fraga-García, P.; Kubbutat, P.; Brammen, M.; Schwaminger, S.; Berensmeier, S. Bare Iron Oxide Nanoparticles for Magnetic Harvesting of Microalgae: From Interaction Behavior to Process Realization. Nanomaterials 2018, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W.; Park, M.S.; Yang, J.-W. Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Salama, E.-S.; Jeon, B.-H.; Kurade, M.B.; Abou-Shanab, R.A.; Govindwar, S.P.; Lee, S.-H.; Yang, I.-S.; Lee, D.S. Harvesting of freshwater microalgae Scenedesmus obliquus and Chlorella vulgaris using acid mine drainage as a cost effective flocculant for biofuel production. Energy Convers. Manag. 2016, 121, 105–112. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Mathew, A.K.; Pandey, A.; Sukumaran, R.K. Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation. Bioresour. Technol. 2016, 213, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.; Lovitt, R.; Flynn, K. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface 2009, 7, 703–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Bharte, S.; Desai, K. Techniques for harvesting, cell disruption and lipid extraction of microalgae for biofuel production. Biofuels 2018, 12, 285–305. [Google Scholar] [CrossRef]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of Microalgae by Flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef] [Green Version]
- Mathimani, T.; Mallick, N. A comprehensive review on harvesting of microalgae for biodiesel—Key challenges and future directions. Renew. Sustain. Energy Rev. 2018, 91, 1103–1120. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Fraga-García, P.; Eigenfeld, M.; Becker, T.M.; Berensmeier, S. Magnetic Separation in Bioprocessing Beyond the Analytical Scale: From Biotechnology to the Food Industry. Front. Bioeng. Biotechnol. 2019, 7, 233. [Google Scholar] [CrossRef]
- Borlido, L.; Azevedo, A.M.; Roque, A.; Aires-Barros, M. Magnetic separations in biotechnology. Biotechnol. Adv. 2013, 31, 1374–1385. [Google Scholar] [CrossRef]
- Ebeler, M.; Pilgram, F.; Wolz, K.; Grim, G.; Franzreb, M. Magnetic Separation on a New Level: Characterization and Performance Prediction of a cGMP Compliant “Rotor-Stator” High-Gradient Magnetic Separator. Biotechnol. J. 2017, 13, 1700448. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-D.; Hiltunen, E.; Li, Z. Using magnetic materials to harvest microalgal biomass: Evaluation of harvesting and detachment efficiency. Environ. Technol. 2017, 40, 1006–1012. [Google Scholar] [CrossRef]
- Boli, E.; Savvidou, M.; Logothetis, D.; Louli, V.; Pappa, G.; Voutsas, E.; Kolisis, F.; Magoulas, K. Magnetic harvesting of marine algae Nannochloropsis oceanica. Sep. Sci. Technol. 2017, 56, 730–737. [Google Scholar] [CrossRef]
- Roth, H.-C.; Schwaminger, S.P.; Schindler, M.; Wagner, F.E.; Berensmeier, S. Influencing factors in the CO-precipitation process of superparamagnetic iron oxide nano particles: A model based study. J. Magn. Magn. Mater. 2015, 377, 81–89. [Google Scholar] [CrossRef]
- Wang, F.; Guo, C.; Yang, L.-R.; Liu, C.-Z. Magnetic mesoporous silica nanoparticles: Fabrication and their laccase immobilization performance. Bioresour. Technol. 2010, 101, 8931–8935. [Google Scholar] [CrossRef]
- Dubey, V.; Kain, V. Synthesis of magnetite by coprecipitation and sintering and its characterization. Mater. Manuf. Process. 2017, 33, 835–839. [Google Scholar] [CrossRef]
- Pospiskova, K.; Prochazkova, G.; Safarik, I. One-step magnetic modification of yeast cells by microwave-synthesized iron oxide microparticles. Lett. Appl. Microbiol. 2013, 56, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; García, P.F.; Merck, G.K.; Bodensteiner, F.A.; Heissler, S.; Günther, S.; Berensmeier, S. Nature of Interactions of Amino Acids with Bare Magnetite Nanoparticles. J. Phys. Chem. C 2015, 119, 23032–23041. [Google Scholar] [CrossRef]
- LKAB. LKAB Year-End Report 2022. 2023. Available online: https://lkab.com/en/press/year-end-report-2022-profits-strong-but-continued-uncertainty (accessed on 28 March 2023).
- Abarca-Cabrera, L.; Xu, L.; Berensmeier, S.; Fraga-García, P. Competition at the Bio-nano Interface: A Protein, a Polysaccharide, and a Fatty Acid Adsorb onto Magnetic Nanoparticles. ACS Appl. Bio Mater. 2022, 6, 146–156. [Google Scholar] [CrossRef]
- Turrina, C.; Berensmeier, S.; Schwaminger, S.P. Bare Iron Oxide Nanoparticles as Drug Delivery Carrier for the Short Cationic Peptide Lasioglossin. Pharmaceuticals 2021, 14, 405. [Google Scholar] [CrossRef]
- Winsett, J.; Moilanen, A.; Paudel, K.; Kamali, S.; Ding, K.; Cribb, W.; Seifu, D.; Neupane, S. Quantitative determination of magnetite and maghemite in iron oxide nanoparticles using Mössbauer spectroscopy. SN Appl. Sci. 2019, 1, 1636. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, T.; Cui, J.; Wei, L. Study on magnetic difference of artificial magnetite and natural magnetite. J. Phys. Conf. Ser. 2020, 1699, 012040. [Google Scholar] [CrossRef]
- Köhler, T.; Feoktystov, A.; Petracic, O.; Kentzinger, E.; Bhatnagar-Schöffmann, T.; Feygenson, M.; Nandakumaran, N.; Landers, J.; Wende, H.; Cervellino, A.; et al. Mechanism of magnetization reduction in iron oxide nanoparticles. Nanoscale 2021, 13, 6965–6976. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [Green Version]
- Omelyanchik, A.; Kamzin, A.; Valiullin, A.; Semenov, V.; Vereshchagin, S.; Volochaev, M.; Dubrovskiy, A.; Sviridova, T.; Kozenkov, I.; Dolan, E.; et al. Iron oxide nanoparticles synthesized by a glycine-modified coprecipitation method: Structure and magnetic properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129090. [Google Scholar] [CrossRef]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse magnetite nanoparticles with nearly ideal saturation magnetization. RSC Adv. 2016, 6, 77452–77464. [Google Scholar] [CrossRef]
- Hadadian, Y.; Masoomi, H.; Dinari, A.; Ryu, C.; Hwang, S.; Kim, S.; Cho, B.K.; Lee, J.Y.; Yoon, J. From Low to High Saturation Magnetization in Magnetite Nanoparticles: The Crucial Role of the Molar Ratios Between the Chemicals. ACS Omega 2022, 7, 15996–16012. [Google Scholar] [CrossRef]
- Schobesberger, M.; Real, B.K.; Meijer, D.; Berensmeier, S.; Fraga-García, P. Natural magnetite ore as a harvesting agent for saline microalgae Microchloropsis salina. Bioresour. Technol. Rep. 2021, 15, 100798. [Google Scholar] [CrossRef]
- Thompson, R.; Oldfield, F. (Eds.) Magnetic properties of natural materials. In Environmental Magnetism; Allen & Unwin: London, UK; Boston, MA, USA; Sydney, Australia, 1986; ISBN 978-94-011-8038-2. [Google Scholar]
- Coey, J.M.D. Magnetic properties of iron in soil iron oxides and clay minerals. In Iron in Soils and Clay Minerals, 217th ed.; Stucki, J.W., Goodman, B.A., Schwertmann, U., Eds.; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1988; pp. 397–466. ISBN 978-94-009-4007-9. [Google Scholar]
- Schädler, T.; Cerbon, D.C.; de Oliveira, L.; Garbe, D.; Brück, T.; Weuster-Botz, D. Production of lipids with Microchloropsis salina in open thin-layer cascade photobioreactors. Bioresour. Technol. 2019, 289, 121682. [Google Scholar] [CrossRef] [PubMed]
- Schädler, T.; Neumann-Cip, A.-C.; Wieland, K.; Glöckler, D.; Haisch, C.; Brück, T.; Weuster-Botz, D. High-Density Microalgae Cultivation in Open Thin-Layer Cascade Photobioreactors with Water Recycling. Appl. Sci. 2020, 10, 3883. [Google Scholar] [CrossRef]
- Retsch GmbH. Planeten-Kugelmühle PM 100. Available online: https://www.retsch.de/de/produkte/zerkleinern/kugelmuehlen/planeten-kugelmuehle-pm-100/funktion-merkmale/ (accessed on 1 February 2023).
- Netzsch Feinmahltechnik GmbH. Grinding System Zeta. Available online: https://grinding.netzsch.com/en/products-and-solutions/wet-grinding/grinding-system-zeta (accessed on 1 February 2023).
- Mucsi, G. A review on mechanical activation and mechanical alloying in stirred media mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Stenger, F.; Mende, S.; Schwedes, J.; Peukert, W. The influence of suspension properties on the grinding behavior of alumina particles in the submicron size range in stirred media mills. Powder Technol. 2005, 156, 103–110. [Google Scholar] [CrossRef]
- Stenger, F. Grenzflächeneffekte bei der Nanozerkleinerung in Rührwerkskugelmühlen. Ph.D. Thesis, Berichte aus der Verfahrenstechnik, Shaker, Aachen, Germany, 2005; Erlangen-Nürnberg University, Aachen, Germany, 2004. [Google Scholar]
- Tokiwa, F.; Imamura, T. Suspension stability of solid particles in the presence of various types of electrolytes. J. Am. Oil Chem. Soc. 1969, 46, 571–574. [Google Scholar] [CrossRef]
- Sørensen, J.S.; Madsen, H.E.L. The influence of magnetism on precipitation of calcium phosphate. J. Cryst. Growth 2000, 216, 399–406. [Google Scholar] [CrossRef]
- Baskar, S.; Ramya, J.R.; Arul, K.T.; Nivethaa, E.; Pillai, V.M.; Kalkura, S.N. Impact of magnetic field on the mineralization of iron doped calcium phosphates. Mater. Chem. Phys. 2018, 218, 166–171. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Yanovska, A.; Stanislavov, A.; Danilchenko, S.; Kalinkevich, A.; Sukhodub, L. Controllability of brushite structural parameters using an applied magnetic field. Mater. Sci. Eng. C 2016, 60, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, C.; Wang, F.; Zheng, S.; Liu, C.-Z. A simple and rapid harvesting method for microalgae by in situ magnetic separation. Bioresour. Technol. 2011, 102, 10047–10051. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae—An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Gerulová, K.; Kucmanová, A.; Sanny, Z.; Garaiová, Z.; Seiler, E.; Čaplovičová, M.; Čaplovič, Ľ.; Palcut, M. Fe3O4-PEI Nanocomposites for Magnetic Harvesting of Chlorella vulgaris, Chlorella ellipsoidea, Microcystis aeruginosa, and Auxenochlorella protothecoides. Nanomaterials 2022, 12, 1786. [Google Scholar] [CrossRef]
- Hu, Y.-R.; Wang, F.; Wang, S.-K.; Liu, C.-Z.; Guo, C. Efficient harvesting of marine microalgae Nannochloropsis maritima using magnetic nanoparticles. Bioresour. Technol. 2013, 138, 387–390. [Google Scholar] [CrossRef]
- Kaveh-Baghbaderani, Y.; Allgayer, R.; Schwaminger, S.P.; Fraga-García, P.; Berensmeier, S. Magnetic Separation of Antibodies with High Binding Capacity by Site-Directed Immobilization of Protein A-Domains to Bare Iron Oxide Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 4956–4963. [Google Scholar] [CrossRef]
- Thomas, J.A.; Schnell, F.; Kaveh-Baghbaderani, Y.; Berensmeier, S.; Schwaminger, S.P. Immunomagnetic Separation of Microorganisms with Iron Oxide Nanoparticles. Chemosensors 2020, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, H.; Cheng, S.; Zhang, W.; Zhang, X. Enhanced Microalgal Harvesting Using Microalgae-Derived Extracellular Polymeric Substance as Flocculation Aid. ACS Sustain. Chem. Eng. 2020, 8, 4069–4075. [Google Scholar] [CrossRef]
- Lv, J.; Guo, B.; Feng, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Integration of wastewater treatment and flocculation for harvesting biomass for lipid production by a newly isolated self-flocculating microalga Scenedesmus rubescens SX. J. Clean. Prod. 2019, 240, 118211. [Google Scholar] [CrossRef]






| Particle Size | Specific BET Surface | Used Concentration | Used Surface | Harvesting Efficiency in % at | |||||
|---|---|---|---|---|---|---|---|---|---|
| d50 (nm) | (m2 g−1) | (g L−1) | (m2 L−1) | pH 4 | pH 6 | pH 8 | pH 10 | pH 11 | |
| MNP | 13–120 | 80 | 0.031 | 2.5 | 5.57 | 6.03 | 22.44 | 74.45 | 99.77 |
| MNO | 254 | 46 | 0.054 | 2.5 | 7.91 | 1.52 | 25.78 | 83.37 | 99.92 |
| MMO EX009 | 5829 | 2.5 | 1 | 2.5 | 0 | 0 | 11.99 | 65.52 | 97.47 |
| MNP | 13–120 | 80 | 0.31 | 25 | 26.88 | 24.83 | 67.72 | 96.90 | 99.75 |
| MNO | 254 | 46 | 0.54 | 25 | 15.95 | 16.34 | 56.29 | 96.92 | 99.98 |
| MMO EX009 | 5829 | 2.5 | 10 | 25 | 10.15 | 9.99 | 36.64 | 86.30 | 99.87 |
| MNP | 13–120 | 80 | 0.1 | 8 | 9.77 | 13.91 | 47.79 | 90.71 | 99.61 |
| MNO | 254 | 46 | 0.1 | 4.6 | 6.84 | 8.77 | 42.72 | 89.33 | 99.67 |
| MMO EX009 | 5829 | 2.5 | 0.1 | 0.25 | 6.13 | 3.75 | 10.98 | 79.95 | 99.47 |
| MNP | 13–120 | 80 | 1 | 80 | 14.75 | 21.05 | 55.95 | 96.29 | 99.50 |
| MNO | 254 | 46 | 1 | 46 | 7.72 | 15.38 | 45.03 | 94.29 | 99.28 |
| MMO EX009 | 5829 | 2.5 | 1 | 2.5 | 0 | 0 | 11.99 | 65.52 | 97.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schobesberger, M.; Helmhagen, S.; Mende, S.; Berensmeier, S.; Fraga-García, P. From Micro to Nano: Grinding Natural Magnetite Ore for Microalgae Harvesting. Magnetochemistry 2023, 9, 149. https://doi.org/10.3390/magnetochemistry9060149
Schobesberger M, Helmhagen S, Mende S, Berensmeier S, Fraga-García P. From Micro to Nano: Grinding Natural Magnetite Ore for Microalgae Harvesting. Magnetochemistry. 2023; 9(6):149. https://doi.org/10.3390/magnetochemistry9060149
Chicago/Turabian StyleSchobesberger, Michael, Simone Helmhagen, Stefan Mende, Sonja Berensmeier, and Paula Fraga-García. 2023. "From Micro to Nano: Grinding Natural Magnetite Ore for Microalgae Harvesting" Magnetochemistry 9, no. 6: 149. https://doi.org/10.3390/magnetochemistry9060149