Single-Molecule Magnets Based on Heteroleptic Terbium(III) Trisphthalocyaninate in Solvent-Free and Solvent-Containing Forms
Abstract
:1. Introduction
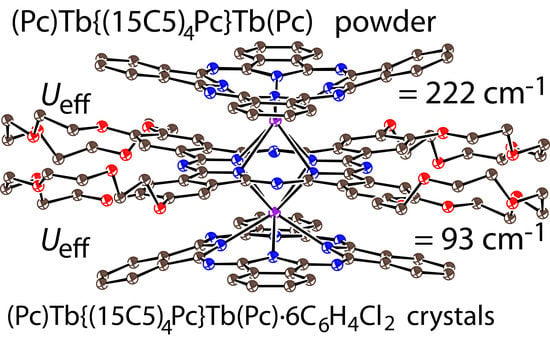
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.1.1. Improved Procedure for the Synthesis of the La(Pc)2 Complex
3.1.2. Improved Procedure for the Synthesis of (Pc)Tb[(15C5)4Pc]Tb(Pc)
3.1.3. Preparation of Single Crystals of (Pc)Tb[(15C5)4Pc]Tb(Pc)⋅6C6H4Cl2 (2)
3.2. General
3.3. X-ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-Y.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- AlDamen, M.A.; Clemente-Juan, J.M.; Coronado, E.; Martí-Gastaldo, C.; Gaita-Ariño, A. Mononuclear Lanthanide Single-Molecule Magnets Based on Polyoxometalates. J. Am. Chem. Soc. 2008, 130, 8874–8875. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit. Angew. Chemie Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Troiani, F.; Affronte, M. Molecular spins for quantum information technologies. Chem. Soc. Rev. 2011, 40, 3119. [Google Scholar] [CrossRef] [Green Version]
- Aromí, G.; Aguilà, D.; Gamez, P.; Luis, F.; Roubeau, O. Design of magnetic coordination complexes for quantum computing. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar] [CrossRef]
- Thiele, S.; Balestro, F.; Ballou, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Electrically driven nuclear spin resonance in single-molecule magnets. Science 2014, 344, 1135–1138. [Google Scholar] [CrossRef]
- Chappert, C.; Fert, A.; Van Dau, F.N. The emergence of spin electronics in data storage. Nat. Mater. 2007, 6, 813–823. [Google Scholar] [CrossRef]
- Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 2011, 40, 3336. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, P. Lanthanide Single-Ion Molecular Magnets. In Lanthanide Single Molecule Magnets; Springer: Berlin/Heidelberg, Germany, 2015; pp. 41–90. [Google Scholar]
- Goodwin, C.A.P. Blocking like it’s hot: A synthetic chemists’ path to high-temperature lanthanide single molecule magnets. Dalton Trans. 2020, 49, 14320–14337. [Google Scholar] [CrossRef] [PubMed]
- Martynov, A.G.; Horii, Y.; Katoh, K.; Bian, Y.; Jiang, J.; Yamashita, M.; Gorbunova, Y.G. Rare-earth based tetrapyrrolic sandwiches: Chemistry, materials and applications. Chem. Soc. Rev. 2022, 51, 9262–9339. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.A.; McClain, K.R.; Reta, D.; Kragskow, J.G.C.; Marchiori, D.A.; Lachman, E.; Choi, E.-S.; Analytis, J.G.; Britt, R.D.; Chilton, N.F.; et al. Ultrahard magnetism from mixed-valence dilanthanide complexes with metal-metal bonding. Science 2022, 375, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Pushkarev, V.E.; Tomilova, L.G.; Nemykin, V.N. Historic overview and new developments in synthetic methods for preparation of the rare-earth tetrapyrrolic complexes. Coord. Chem. Rev. 2016, 319, 110–179. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, N.; Sugita, M.; Tanaka, N.; Ishikawa, T.; Koshihara, S.; Kaizu, Y. Upward temperature shift of the intrinsic phase lag of the magnetization of Bis(phthalocyaninato)terbium by ligand oxidation creating an S = 1/2 spin. Inorg. Chem. 2004, 43, 5498–5500. [Google Scholar] [CrossRef]
- Ganivet, C.R.; Ballesteros, B.; de la Torre, G.; Clemente-Juan, J.M.; Coronado, E.; Torres, T.; de la Torre, G.; Clemente-Juan, J.M.; Coronado, E.; Torres, T.; et al. Influence of Peripheral Substitution on the Magnetic Behavior of Single-Ion Magnets Based on Homo- and Heteroleptic Tb III Bis(phthalocyaninate). Chem. Eur. J. 2013, 19, 1457–1465. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, F.; Chen, X.; Dong, B.; Wang, K.; Jiang, S.; Wang, C.; Chen, X.; Qi, D.; Sun, H.; et al. A New Bis(phthalocyaninato) Terbium Single-Ion Magnet with an Overall Excellent Magnetic Performance. Inorg. Chem. 2017, 56, 13889–13896. [Google Scholar] [CrossRef]
- Ishikawa, N.; Otsuka, S.; Kaizu, Y. The Effect of the f–f Interaction on the Dynamic Magnetism of a Coupled 4f8 System in a Dinuclear Terbium Complex with Phthalocyanines. Angew. Chemie Int. Ed. 2005, 44, 731–733. [Google Scholar] [CrossRef]
- Katoh, K.; Kajiwara, T.; Nakano, M.; Nakazawa, Y.; Wernsdorfer, W.; Ishikawa, N.; Breedlove, B.K.; Yamashita, M. Magnetic Relaxation of Single-Molecule Magnets in an External Magnetic Field: An Ising Dimer of a Terbium(III)-Phthalocyaninate Triple-Decker Complex. Chem. Eur. J. 2011, 17, 117–122. [Google Scholar] [CrossRef]
- Katoh, K.; Horii, Y.; Yasuda, N.; Wernsdorfer, W.; Toriumi, K.; Breedlove, B.K.; Yamashita, M. Multiple-decker phthalocyaninato dinuclear lanthanoid(iii) single-molecule magnets with dual-magnetic relaxation processes. Dalton Trans. 2012, 41, 13582–13600. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, S.; Fuyuhiro, A.; Fukuda, T.; Ishikawa, N. Dinuclear single-molecule magnets with porphyrin–phthalocyanine mixed triple-decker ligand systems giving SAP and SP coordination polyhedra. Chem. Commun. 2012, 48, 5337. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Controlling the Dipole–Dipole Interactions between Terbium(III) Phthalocyaninato Triple-Decker Moieties through Spatial Control Using a Fused Phthalocyaninato Ligand. Inorg. Chem. 2013, 52, 13555–13561. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Katoh, K.; Yasuda, N.; Breedlove, B.K.; Yamashita, M. Effects of f-f interactions on the single-molecule magnet properties of terbium(iii)-phthalocyaninato quintuple-decker complexes. Inorg. Chem. 2015, 54, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, R.J.; Polovkova, M.A.; Martynov, A.G.; Gorbunova, Y.G.; Murugesu, M. Impact of the coordination environment on the magnetic properties of single-molecule magnets based on homo- and hetero-dinuclear terbium(III) heteroleptic tris(crownphthalocyaninate). Dalton Trans. 2016, 45, 9320–9327. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.W.; Bian, Y.; Gao, S.; Jiang, J. Single-molecule magnetism of tetrapyrrole lanthanide compounds with sandwich multiple-decker structures. Coord. Chem. Rev. 2016, 306, 195–216. [Google Scholar] [CrossRef]
- Martynov, A.G.; Polovkova, M.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Redox-Triggered Switching of Conformational State in Triple-Decker Lanthanide Phthalocyaninates. Molecules 2022, 27, 6498. [Google Scholar] [CrossRef]
- Martynov, A.G.; Polovkova, M.A.; Berezhnoy, G.S.; Sinelshchikova, A.A.; Khrustalev, V.N.; Birin, K.P.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Heteroleptic Crown-Substituted Tris(phthalocyaninates) as Dynamic Supramolecular Scaffolds with Switchable Rotational States and Tunable Magnetic Properties. Inorg. Chem. 2021, 60, 9110–9121. [Google Scholar] [CrossRef]
- Martynov, A.G.; Zubareva, O.V.; Gorbunova, Y.G.; Sakharov, S.G.; Nefedov, S.E.; Dolgushin, F.M.; Tsivadze, A.Y. Diphthalocyaninatolanthanum as a New Phthalocyaninato-Dianion Donor for the Synthesis of Heteroleptic Triple-Decker Rare Earth Element Crown-Phthalocyaninato Complexes. Eur. J. Inorg. Chem. 2007, 2007, 4800–4807. [Google Scholar] [CrossRef]
- Martynov, A.G.; Bykov, A.V.; Gorbunova, Y.G.; Tsivadze, A.Y. Spectrophotometric study of the cation-induced dimerization of heteroleptic terbium(III) tetra-15-crown-5-bisphthalocyaninate. Russ. Chem. Bull. 2018, 67, 2195–2200. [Google Scholar] [CrossRef]
- Martynov, A.G.; Gorbunova, Y.G. Heteroleptic phthalocyaninato-[tetra(15-crown-5)phthalocyaninato] lanthanides(III) double-deckers: Synthesis and cation-induced supramolecular dimerisation. Inorg. Chim. Acta 2007, 360, 122–130. [Google Scholar] [CrossRef]
- Ishikawa, N.; Iino, T.; Kaizu, Y. Interaction between f-electronic systems in dinuclear lanthanide complexes with phthalocyanines. J. Am. Chem. Soc. 2002, 124, 11440–11447. [Google Scholar] [CrossRef]
- Konarev, D.V.; Khasanov, S.S.; Batov, M.S.; Martynov, A.G.; Nefedova, I.V.; Gorbunova, Y.G.; Otsuka, A.; Yamochi, H.; Kitagawa, H.; Lyubovskaya, R.N. Effect of One- and Two-Electron Reduction of Terbium(III) Double-Decker Phthalocyanine on Single-Ion Magnet Behavior and NIR Absorption. Inorg. Chem. 2019, 58, 5058–5068. [Google Scholar] [CrossRef] [PubMed]
- Yamabayashi, T.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Molecular orientation of a terbium(III)-phthalocyaninato double-decker complex for effective suppression of quantum tunneling of the magnetization. Molecules 2017, 22, 999. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.-Y.; Cui, L.; Li, J.; Yu, F.; Song, Y.; Zhang, Y.-Q.; Zuo, J.-L.; Kurmoo, M. Modulating Single-Molecule Magnetic Behavior of a Dinuclear Erbium(III) Complex by Solvent Exchange. Inorg. Chem. 2017, 56, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Damjanović, M.; Ajayakumar, M.R.; Katoh, K.; Kitagawa, Y.; Chibotaru, L.; Ungur, L.; Mas-Torrent, M.; Wernsdorfer, W.; Breedlove, B.K.; et al. Highly Oxidized States of Phthalocyaninato Terbium(III) Multiple-Decker Complexes Showing Structural Deformations, Biradical Properties and Decreases in Magnetic Anisotropy. Chem. A Eur. J. 2020, 26, 8621–8630. [Google Scholar] [CrossRef]
- Martynov, A.G.; Berezhnoy, G.S.; Safonova, E.A.; Polovkova, M.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Aromatic Nucleophilic Substitution as a Side Process in the Synthesis of Alkoxy- and Crown-Substituted (Na)phthalocyanines. Macroheterocycles 2019, 12, 75–81. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]





| Compound | 1 | 2 |
|---|---|---|
| The Tb-Tb distance, Å | - | 3.4667 (3) |
| Twist angle between inner and outer planes, ° | - | 42.6 |
| Ueff, cm−1 | 222 | 93 |
| Blocking temperature, K | ~6 | ~6 |
| Type of coupling between TbIII spins | ferromagnetic | ferromagnetic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraonov, M.A.; Martynov, A.G.; Polovkova, M.A.; Khasanov, S.S.; Gorbunova, Y.G.; Tsivadze, A.Y.; Otsuka, A.; Yamochi, H.; Kitagawa, H.; Konarev, D.V. Single-Molecule Magnets Based on Heteroleptic Terbium(III) Trisphthalocyaninate in Solvent-Free and Solvent-Containing Forms. Magnetochemistry 2023, 9, 36. https://doi.org/10.3390/magnetochemistry9020036
Faraonov MA, Martynov AG, Polovkova MA, Khasanov SS, Gorbunova YG, Tsivadze AY, Otsuka A, Yamochi H, Kitagawa H, Konarev DV. Single-Molecule Magnets Based on Heteroleptic Terbium(III) Trisphthalocyaninate in Solvent-Free and Solvent-Containing Forms. Magnetochemistry. 2023; 9(2):36. https://doi.org/10.3390/magnetochemistry9020036
Chicago/Turabian StyleFaraonov, Maxim A., Alexander G. Martynov, Marina A. Polovkova, Salavat S. Khasanov, Yulia G. Gorbunova, Aslan Yu. Tsivadze, Akihiro Otsuka, Hideki Yamochi, Hiroshi Kitagawa, and Dmitri V. Konarev. 2023. "Single-Molecule Magnets Based on Heteroleptic Terbium(III) Trisphthalocyaninate in Solvent-Free and Solvent-Containing Forms" Magnetochemistry 9, no. 2: 36. https://doi.org/10.3390/magnetochemistry9020036