Comparison of the Growth, Physio-Biochemical Characteristics, and Quality Indices in Soilless-Grown Strawberries under Greenhouse and Open-Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Cropping System
2.2. Growth and Morphological Parameters
2.3. Biochemical and Physiological Parameters
2.3.1. Photosynthetic Pigments
2.3.2. Physiological Parameters
2.3.3. Biochemical Parameters
2.4. Statistical Analysis
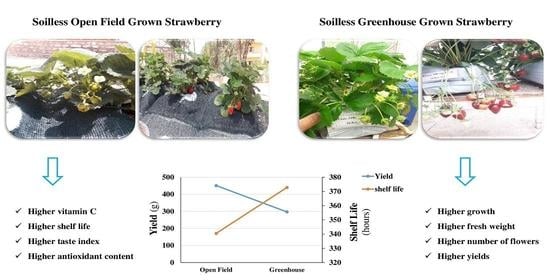
3. Results and Discussion
3.1. Growth and Morphological Parameters
3.2. Biochemical and Physiological Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pandey, S.; Sinh, J.; Singh, S.K.; Mourya, I.B. Influence of growing environment on growth, yield and chemical composition of strawberry (Fragaria × ananassa) fruits under open vs. naturally ventilated polyhouse conditions. Indian J. Agric. Sci. 2015, 85, 1540–1545. [Google Scholar]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, B.; Naeimi, A.; Badsar, M. Designing an integrated model for strawberry growers’ behavior toward implementation of good agricultural practices in Iran. Environ. Dev. Sustain. 2021, 24, 10924–10944. [Google Scholar] [CrossRef]
- Cormier, J.; Depardieu, C.; Letourneau, G.; Boily, C.; Gallichand, J.; Caron, J. Tensiometer-based irrigation scheduling and water use efficiency of field-grown strawberries. Agron. J. 2020, 112, 2581–2597. [Google Scholar] [CrossRef]
- Maas, J.L. Strawberry disease management. In Diseases of Fruits and Vegetables; Springer: Berlin/Heidelberg, Germany, 2004; Volume II, pp. 441–483. [Google Scholar]
- Jabbar, A.; Tehranifar, A.; Shoor, M.; Nemati, S.H. Effect of different media on some growth, flowering and biochemical parameters of two cultivars of gladiolus (Gladiolus grandiflorus L.) under soilless conditions. J. Ornam. Plants 2018, 8, 205–215. [Google Scholar]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: A review. Sci. Total. Environ. 2020, 698, 134154. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Baruzzi, G.; Massetani, F.; Faedi, W. Strawberry production in forced and protected culture in Europe as a response to climate change. Can. J. Plant Sci. 2012, 92, 1021–1036. [Google Scholar] [CrossRef]
- Thakur, N.; Nigam, M.; Awasthi, G.; Shukla, A.; Shah, A.A.; Negi, N.; Khan, S.A.; Casini, R.; Elansary, H.O. Synergistic soil-less medium for enhanced yield of crops: A step towards incorporating genomic tools for attaining net zero hunger. Funct. Integr. Genom. 2023, 23, 86. [Google Scholar] [CrossRef]
- Palencia, P.; Martínez, F.; Vázquez, M.A. Oxyfertigation and Transplanting Conditions of Strawberries. Agronomy 2021, 11, 2513. [Google Scholar] [CrossRef]
- Cecatto, A.P.; Calvete, E.O.; Nienow, A.A.; da Costa, R.C.; Mendonça, H.F.C.; Pazzinato, A.C. Culture systems in the production and quality of strawberry cultivars. Acta Sci. Agron. 2013, 35, 471–478. [Google Scholar] [CrossRef]
- Maher, M.; Shylla, B.; Sharma, D.; Sharma, U.; Kuchay, M. Yield and quality of polyhouse grown strawberries as affected by soilless media and jeevamrit. Int. J. Chem. Stud. 2020, 8, 585–589. [Google Scholar] [CrossRef]
- Del Bubba, M.; Checchini, L.; Chiuminatto, U.; Doumett, S.; Fibbi, D.; Giordani, E. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of polyphenolic composition of four cultivars of Fragaria vesca L. berries and their comparative evaluation. J. Mass Spectrom. 2012, 47, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Gonnella, M.; Renna, M. The Evolution of soilless systems towards ecological sustainability in the perspective of a circular economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Nicola, S.; Savvas, D.; Voogt, W. Soilless cultivation through an intensive crop production scheme. Management strategies, challenges and future directions. Front. Plant Sci. 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Bassal, A.M.; Leonardi, C.; Giuffrida, F.; Colla, G. Vegetable quality as affected by genetic, agronomic and environmental factors. J. Food Agric. Environ. 2012, 10, 680–688. [Google Scholar]
- Weston, L.; Barth, M. Preharvest factors affecting postharvest quality of vegetables. HortScience 1997, 32, 812–816. [Google Scholar] [CrossRef]
- Caruso, G.; Villari, A.; Villari, G. Quality characteristics of Fragaria vesca L. fruits influenced by NFT solution EC and shading. In Proceedings of the South Pacific Soilless Culture Conference (SPSCC 648), Palmerston North, New Zealand, 10–13 February 2003. [Google Scholar]
- Shinohara, Y. Growing Conditions and Quality of Vegetables: Effect of Light and Fertilizer Conditions on the Ascorbic Acid Content of Vegetables; Memoirs of Institute of Agriculture and Forestry-University of Tsukuba, Agricultural and Forestry Science: Ibaraki, Japan, 1987. [Google Scholar]
- Krishna, P.; Pandey, G.; Thomas, R.; Parks, S. Improving Blueberry Fruit Nutritional Quality through Physiological and Genetic Interventions: A Review of Current Research and Future Directions. Antioxidants 2023, 12, 810. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Urban, J.J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef]
- Tuzet, A.; Perrier, A.; Leuning, R. A coupled model of stomatal conductance, photosynthesis and transpiration. Plant Cell Environ. 2003, 26, 1097–1116. [Google Scholar] [CrossRef]
- Crawford, A.J.; McLachlan, D.H.; Hetherington, A.M.; Franklin, K.A. High temperature exposure increases plant cooling capacity. Curr. Biol. 2012, 22, R396–R397. [Google Scholar] [CrossRef] [PubMed]
- Feller, U.; Vaseva, I.I. Extreme climatic events: Impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2014, 2, 39. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Trishchenko, A.P.; Barr, A.G.; Black, T.A.; McCaughey, H. Modeling the Response of Canopy Stomatal Conductance to Humidity. J. Hydrometeorol. 2009, 10, 521–532. [Google Scholar] [CrossRef]
- Fanourakis, D.; Bouranis, D.; Giday, H.; Carvalho, D.R.; Nejad, A.R.; Ottosen, C.-O. Improving stomatal functioning at elevated growth air humidity: A review. J. Plant Physiol. 2016, 207, 51–60. [Google Scholar] [CrossRef]
- Gago, J.; de Menezes Daloso, D.; Figueroa, C.M.; Flexas, J.; Fernie, A.R.; Nikoloski, Z. Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: A multispecies meta-analysis approach. Plant Physiol. 2016, 171, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Mariani, L. Agronomic management for enhancing plant tolerance to abiotic stresses: High and low values of temperature, light intensity, and relative humidity. Horticulturae 2018, 4, 21. [Google Scholar] [CrossRef]
- Caruso, G.; Villari, G.; Melchionna, G.; Conti, S. Effects of cultural cycles and nutrient solutions on plant growth, yield and fruit quality of alpine strawberry (Fragaria vesca L.) grown in hydroponics. Sci. Hortic. 2011, 129, 479–485. [Google Scholar] [CrossRef]
- Døving, A.; Måge, F. Methods of Testing Strawberry Fruit Firmness. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2002, 52, 43–51. [Google Scholar] [CrossRef]
- Smith, J.H.; Benitez, A. Chlorophylls: Analysis in plant materials. In Modern Methods of Plant Analysis/Moderne Methoden der Pflanzenanalyse; Springer: Berlin/Heidelberg, Germany, 1955; pp. 142–196. [Google Scholar]
- Berger, S. Metoda ilościowego oznaczania karotenu (prowitamina A) i sumy karotenów w niektórych produktach roślinnych. Rocz. Państwowego Zakładu Hig. 1953, 4, 473–479. [Google Scholar]
- Lutts, S.; Kinet, J.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Nguyen, H.T.; Holaday, A.S. Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Sci. 1990, 30, 105–111. [Google Scholar] [CrossRef]
- Jungsakulrujirek, S.; Noomhorm, A. Effect of harvesting time and fruit size on titratable acidity, soluble solid and distribution of limonin in Thai tangerine juice. Int. J. Food Sci. Technol. 1998, 33, 367–374. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z.; Guan, L.; Gaughan, G.; Lin, G. Antioxidant Enzymes Regulate Reactive Oxygen Species during Pod Elongation in Pisum sativum and Brassica chinensis. PLoS ONE 2014, 9, e87588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, S.; Li, Z.; Liu, N.; Li, L.; Luo, L.; Peng, T.; Liu, W. Effects of the infestation by Actinote thalia pyrrha (Fabricius) on the physiological indexes of Mikania micrantha leaves. Acta Ecol. Sin. 2006, 26, 1330–1336. [Google Scholar] [CrossRef]
- Zeng, S.-X.; Wang, Y.-R.; Liu, H.-X. Some enzymatic reactions related to chlorophyll degradation in cucumber cotyledons under chilling in the light. Acta Phytophysiol Sin 1991, 17, 177–182. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Hosu, A.; Cristea, V.-M.; Cimpoiu, C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines: Prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 152–178. [Google Scholar]
- Rahmatian, A.; Delshad, M.; Salehi, R. Effect of grafting on growth, yield and fruit quality of single and double stemmed tomato plants grown hydroponically. Hortic. Environ. Biotechnol. 2014, 55, 115–119. [Google Scholar] [CrossRef]
- Zucker, M. Sequential induction of phenylalanine ammonia-lyase and a lyase-inactivating system in potato tuber disks. Plant Physiol. 1968, 43, 365–374. [Google Scholar] [CrossRef]
- Cocco, C.; Andriolo, J.L.; Cardoso, F.L.; Erpen, L.; Schmitt, O.J. Crown size and transplant type on the strawberry yield. Sci. Agricola 2011, 68, 489–493. [Google Scholar] [CrossRef]
- Grijalba, C.M.; Pérez-Trujillo, M.M.; Ruiz, D.; Ferrucho, A.M. Strawberry yields with high-tunnel and open-field cultivations and the relationship with vegetative and reproductive plant characteristics. Agron. Colomb. 2015, 33, 147–154. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Photosynthetic capacity, photochemical efficiency and chlorophyll content of three varieties of Labisia pumila Benth. Exposed to open field and greenhouse growing conditions. Acta Physiol. Plant 2011, 33, 2179–2185. [Google Scholar] [CrossRef]
- Adir, N.; Zer, H.; Shochat, S.; Ohad, I. Photoinhibition—A historical perspective. Photosynth. Res. 2003, 76, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Liu, X. Photosynthetic characteristics of linze jujube in conditions of high temperature and irradiation. Sci. Hortic. 2005, 104, 339–350. [Google Scholar] [CrossRef]
- Habermann, E.; De Oliveira, E.A.D.; Contin, D.R.; Martin, J.A.B.S.; Curtarelli, L.; Gonzalez-Meler, M.; Martinez, C.A. Stomatal Development and Conductance of a Tropical Forage Legume Are Regulated by Elevated [CO2] Under Moderate Warming. Front. Plant Sci. 2019, 10, 609. [Google Scholar] [CrossRef]
- Tanentzap, F.M.; Stempel, A.; Ryser, P. Reliability of leaf relative water content (RWC) measurements after storage: Consequences for in situ measurements. Botany 2015, 93, 535–541. [Google Scholar] [CrossRef]
- Katsoulas, N.; Bari, A.; Papaioannou, C. Plant Responses to UV Blocking Greenhouse Covering Materials: A Review. Agronomy 2020, 10, 1021. [Google Scholar] [CrossRef]
- Hideg, É.; Strid, Å. The effects of UV-B on the biochemistry and metabolism of plants. In UV-B Radiation and Plant Life; CAB International: Oxfordshire, UK, 2017; pp. 90–110. [Google Scholar]
- Han, W.; Yang, Z.; Huang, L.; Sun, C.; Yu, X.; Zhao, M. Fuzzy comprehensive evaluation of the effects of relative air humidity on the morpho-physiological traits of Pakchoi (Brassica chinensis L.) under high temperature. Sci. Hortic. 2019, 246, 971–978. [Google Scholar] [CrossRef]
- Haghshenas, M.; Nazarideljou, M.J.; Shokoohian, A. Phytochemical and Quality Attributes of Strawberry Fruit under Osmotic Stress of Nutrient Solution and Foliar Application of Putrescine and Salicylic Acid. J. Hortic. Sci. 2020, 7, 263–278. [Google Scholar]
- Scandalios, J.G. Response of Plant Antioxidant Defense Genes to Environmental Stress. Adv. Genet. 1990, 28, 1–41. [Google Scholar]
- Del Río, L.A.; Corpas, F.J.; Lopez-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–26. [Google Scholar]
- Ślesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kuk, Y.I.; Shin, J.S.; Burgos, N.R.; Hwang, T.E.; Han, O.; Cho, B.H.; Jung, S.; Guh, J.O. Antioxidative Enzymes Offer Protection from Chilling Damage in Rice Plants. Crop Sci. 2003, 43, 2109–2117. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, H.; Mittelstrass, K.; Kschieschan, S.; Bender, J.; Weigel, H.-J.; Overmyer, K.; Kangasjärvi, J.; Sandermann, H.; Langebartels, C. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ. 2002, 25, 717–726. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.D.; Van Breusegem, F. Hydrogen peroxide—A central hub for information flow in plant cells. AoB Plants 2012, 2012, pls014. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef]
- Ahmed, C.B.; Rouina, B.B.; Sensoy, S.; Boukhris, M.; Abdallah, F.B. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Thiaw, S.; Hall, A.E. Comparison of selection for either leaf-electrolyte-leakage or pod set in enhancing heat tolerance and grain yield of cowpea. Field Crops Res. 2004, 86, 239–253. [Google Scholar] [CrossRef]
- Bhattarai, S.; Harvey, J.T.; Djidonou, D.; Leskovar, D.I. Exploring Morpho-Physiological Variation for Heat Stress Tolerance in Tomato. Plants 2021, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Halbwirth, H.; Puhi, I.; Hass, U.; Jezik, K.; Treutter, D.; Stich, K. Two-phase flavonoid formation in developing strawberry (Fragaria × ananassa) fruit. J. Agric. Food Chem. 2006, 54, 1479–1485. [Google Scholar] [CrossRef]
- Botella, M.; Hernández, V.; Mestre, T.; Hellín, P.; García-Legaz, M.F.; Rivero, R.M.; Martínez, V.; Fenoll, J.; Flores, P. Bioactive Compounds of Tomato Fruit in Response to Salinity, Heat and Their Combination. Agriculture 2021, 11, 534. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Veronneau, P.-Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Irradiation Increased the Level of Polyphenol Accumulation and Flavonoid Pathway Gene Expression in Strawberry Fruit. J. Agric. Food Chem. 2017, 65, 9970–9979. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Gao, Q.; Chen, J.-X. Effects of day and night temperature difference on growth, development, yield and fruit quality of tomatoes. Ying Yong Sheng Tai Xue Bao 2015, 26, 2700–2706. [Google Scholar]
- Wu, X.; Han, W.; Yang, Z.; Zhang, Y.; Zheng, Y. The difference in temperature between day and night affects the strawberry soluble sugar content by influencing the photosynthesis, respiration and sucrose phosphatase synthase. Hortic. Sci. 2021, 48, 174–182. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Galal, F.H.; Seufi, A.M. Effect of extreme temperature changes on phenolic, flavonoid contents and antioxidant activity of tomato seedlings (Solanum lycopersicum L.). PeerJ 2021, 9, e11193. [Google Scholar] [CrossRef]
- Mamat, S.F.; Azizan, K.A.; Baharum, S.N.; Noor, N.M.; Aizat, W.M. GC-MS and LC-MS analyses reveal the distribution of primary and secondary metabolites in mangosteen (Garcinia mangostana Linn.) fruit during ripening. Sci. Hortic. 2020, 262, 109004. [Google Scholar] [CrossRef]
- Ordidge, M.; García-Macías, P.; Battey, N.H.; Gordon, M.H.; John, P.; A Lovegrove, J.; Vysini, E.; Wagstaffe, A.; Hadley, P. Development of colour and firmness in strawberry crops is UV light sensitive, but colour is not a good predictor of several quality parameters. J. Sci. Food Agric. 2012, 92, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- García-Macías, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem. 2007, 55, 10168–10172. [Google Scholar] [CrossRef] [PubMed]





| Month | Culture Condition | Day Temperature (°C) | Night Temperature (°C) | Relative Humidity (%) | Light Intensity (µmol m−2 s−1) |
|---|---|---|---|---|---|
| April | SG | 18 | 16 | 45 | 120.4 |
| SOF | 13 | 7 | 60 | 314.8 | |
| May | SG | 20 | 17 | 53 | 177.8 |
| SOF | 22 | 15 | 38 | 796.3 | |
| June | SG | 21 | 19 | 51 | 388.9 |
| SOF | 25.8 | 17 | 14 | 1203.7 | |
| July | SG | 24 | 20 | 50 | 481.5 |
| SOF | 28 | 19 | 10 | 1314.8 | |
| August | SG | 25 | 21 | 45 | 425.9 |
| SOF | 30 | 19 | 5 | 1166.7 | |
| September | SG | 23 | 20 | 51 | 259.3 |
| SOF | 25 | 16 | 7 | 944.4 |
| EC (dS/m) | Macronutrients (mM) | Micronutrients (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | Fe | Cu | Mn | Zn | B | Mo | |
| 1.3 | 6.1 | 0.5 | 2.6 | 2 | 1.6 | 1.3 | 45 | 20 | 25 | 38 | 12 | 1 |
| Different Morphological Characteristics | Greenhouse | Open Field |
|---|---|---|
| Yield (g plant−1) | 451.1 ± 80 a * | 279.7 ± 49.7 b |
| Plant fresh weight (g plant−1) | 254.8 ± 49.8 a | 170.3 ± 37.4 b |
| Number of fruit (per plant) | 26.8 ± 4.6 a | 20.2 ± 2.3 b |
| Fruit firmness (Newton m−2) | 1.5 ± 0.3 b | 1.6 ± 0.3 a |
| Single fruit fresh weight (g fruit−1) | 16.9 ± 1.3 a | 13.8 ± 1.5 b |
| Crown number (unit plant−1) | 3 ± 1.1 a | 3.3 ± 1.1 a |
| Crown diameter (mm) | 21.5 ± 5.6 a | 16.3 ± 4.3 b |
| Root length (mm) | 38.3 ± 6 a | 31 ± 5.8 b |
| Root volume (m3) | 46.9 ± 10.3 a | 35.1 ± 9 b |
| Fruit diameter (mm) | 32.5 ± 1.2 a | 25.3 ± 1.1 b |
| Fruit length (mm) | 35.9 ± 2.5 a | 32.6 ± 2.2 b |
| Fruit dry matter (%) | 11.4 ± 1.8 b | 13.7 ± 1.3 a |
| Unmarketable fruit (%) | 2.7 ± 0.1 b | 4.8 ± 0.5 a |
| Fruit shelf-life at 2 °C (hours) | 340.5 ± 68 b | 372.8 ± 14 a |
| Fruit shelf-life at 22 °C (hours) | 60 ± 21 b | 80 ± 15.7 a |
| Fruit weight loss at 2 °C (%) | 11.8 ± 2.4 a | 6 ± 1.4 b |
| Photosynthetic Pigments | Open Field | Greenhouse |
|---|---|---|
| Chlorophyll a (mg g−1) | 21.06 ± 1.9 b * | 23.6 ± 1.1 a |
| Chlorophyll b (mg g−1) | 5.53 ± 1.1 b | 7.03 ± 0.76 a |
| Total chlorophyll (a + b) (mg g−1) | 26.59 ± 2.6 b | 30.63 ± 2.1 a |
| Carotenoid (mg g−1) | 1.97 ± 0.85 b | 3.67 ± 1.2 a |
| Organ | Different Physio-Biochemical Characteristics | Greenhouse | Open Field |
|---|---|---|---|
| Leaf | Proline (μg mL−1) | 3.57 ± 0.8 b * | 5.5 ± 1.8 a |
| Ion leakage (%) | 51.68 ± 6.37 b | 64.5 ± 4.6 a | |
| Stomatal conductivity (mol H2O m−2 s−1) | 412.2 ± 54.8 a | 308.8 ± 39.6 b | |
| Relative water content (%) | 50.17 ± 3.9 a | 43.4 ± 3.1 b | |
| Fruit | TSS (°Brix) | 10.64 ± 1.4 b | 13.6 ± 0.9 a |
| DPPH (%) | 59.5 ± 11.5 b | 72 ± 8.7 a | |
| TSS:TA ratio | 11.16 ± 1.5 b | 13.28 ± 1.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahim Doust, J.; Nazarideljou, M.J.; Arshad, M.; Ferrante, A. Comparison of the Growth, Physio-Biochemical Characteristics, and Quality Indices in Soilless-Grown Strawberries under Greenhouse and Open-Field Conditions. Horticulturae 2023, 9, 774. https://doi.org/10.3390/horticulturae9070774
Rahim Doust J, Nazarideljou MJ, Arshad M, Ferrante A. Comparison of the Growth, Physio-Biochemical Characteristics, and Quality Indices in Soilless-Grown Strawberries under Greenhouse and Open-Field Conditions. Horticulturae. 2023; 9(7):774. https://doi.org/10.3390/horticulturae9070774
Chicago/Turabian StyleRahim Doust, Jalil, Mohammad Javad Nazarideljou, Mousa Arshad, and Antonio Ferrante. 2023. "Comparison of the Growth, Physio-Biochemical Characteristics, and Quality Indices in Soilless-Grown Strawberries under Greenhouse and Open-Field Conditions" Horticulturae 9, no. 7: 774. https://doi.org/10.3390/horticulturae9070774