In Vitro Propagation of Three Populations of the Endangered, Greek Endemic Cerastium candidissimum and Short-Term Storability of Alginate-Encapsulated Shoot Explants for Exploitation and Conservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
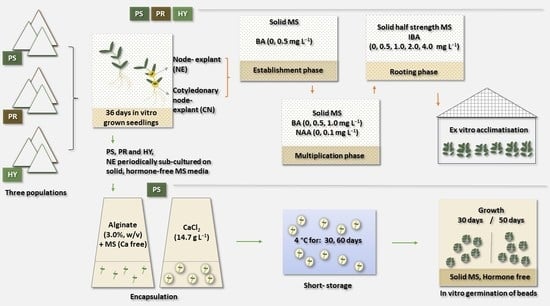
2.2. Establishment of Initial Cultures
2.3. Multiplication Phase
2.4. In Vitro Rooting and Acclimatisation
2.5. Encapsulation
2.6. In Vitro Culture Conditions and Data Collection
2.7. Statistical Analysis
3. Results
3.1. Establishment of Initial Cultures
3.2. Multiplication
3.3. Rooting Phase and Acclimatisation
3.4. Shoot Growth from Beads
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strid, A. Mountain Flora of Greece; Cambridge University Press: Cambridge, UK, 1986; Volume 1, pp. 110–122. [Google Scholar]
- Khalaf, M.K. Biosystematic studies in Cerastium tomentosum group (Caryophyllaceae). Ph.D. Thesis, University of Leicester, Leicester, UK, 1993. Available online: https://hdl.handle.net/2381/35321 (accessed on 23 December 2022).
- Darras, A. Overview of the Dynamic Role of Specialty Cut Flowers in the International Cut Flower Market. Horticulturae 2021, 7, 51. [Google Scholar] [CrossRef]
- Klett, J.E.; Wilson, C.R.; Feucht, J.R. Xeriscaping: Ground Cover Plants. Gardening Series. Yard 2007, no. 7.230. Available online: https://extension.colostate.edu/docs/pubs/garden/07230.pdf (accessed on 2 December 2022).
- Krigas, N.; Menteli, V.; Vokou, D. The electronic trade in Greek endemic plants: Biodiversity, commercial and legal aspects. Econ. Bot. 2014, 68, 85–95. [Google Scholar] [CrossRef]
- Cheminal, A.; Kokkoris, I.P.; Zotos, A.; Strid, A.; Dimopoulos, P. Assessing the Ecosystem Services Potential of Endemic Floras: A Systematic Review on the Greek Endemics of Peloponnese. Sustainability 2022, 14, 5926. [Google Scholar] [CrossRef]
- Couladis, M.; Tzakou, O. Volatile constituents of Cerastium candidissimum Corr. from Greece. J. Essent. Oil Res. 2000, 12, 691–692. [Google Scholar] [CrossRef]
- Lazari, D.M.; Skaltsa, H.D.; Constantinidis, T. Volatile constituents of Cerastium candidissimum, a Greek endemic species. Flavour Fragr. J. 2000, 15, 174–176. [Google Scholar] [CrossRef]
- Stamatis, G.; Kyriazopoulos, P.; Golegou, S.; Basayiannis, A.; Skaltsas, S.; Skaltsa, H. In vitro anti-Helicobacter pylori activity of Greek herbal medicines. J. Ethnopharmacol. 2003, 88, 175–179. [Google Scholar] [CrossRef]
- Maloupa, E.; Krigas, N.; Grigoriadou, K.; Lazari, D.; Tsoktouridis, G. Conservation strategies for native plant species and their sustainable exploitation: Case of the Balkan Botanic Garden of Kroussia, N. Greece. In Floriculture Ornamental Plant Biotechnology: Advances and Topical Issues, 1st ed.; Teixeira da Silva, J.A., Ed.; Global Science Books: Isleworth, UK, 2008; Volume V, pp. 37–56. [Google Scholar]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Cristea, V.; Besenyei, E.; Jarda, L.; Farkas, A.; Marcu, D.; Clapa, D.; Halmagyl, A.; Butiuc-Keul, A. In-situ genetic variability and micropropagation of Cerastium banaticum (Rochel) Heuff. (Caryophyllaceae)—A rare and endemic species from Romania. Acta Biol. Crac. Ser. Bot. 2019, 61, 65–74. [Google Scholar] [CrossRef]
- Papafotiou, M.; Bertsouklis, K.F.; Trigka, M. Micropropagation of Arbutus unedo, A. andrachne, and their natural hybrid, A. x andrachnoides from seedling explants. J. Hortic. Sci. Biotechnol. 2013, 88, 768–775. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Theodorou, P.; Aretaki, P.-E. In vitro Propagation of the Mount Parnitha Endangered Species Sideritis raeseri subsp. Attica. Horticulturae 2022, 8, 1114. [Google Scholar] [CrossRef]
- Dalal, N.V.; Rai, V.R. In vitro propagation of Oroxylum indicum Vent. a medicinally important forest tree. J. For. Res. 2004, 9, 61–65. [Google Scholar] [CrossRef]
- Trigka, M.; Papafotiou, M. In vitro propagation of Anthyllis barba-jovis from seedling tissues. Acta Hortic. 2017, 1189, 473–748. [Google Scholar] [CrossRef]
- Song, H.; Mao, W.; Shang, Y.; Zhou, W.; Li, P.; Chen, X. A regeneration system using cotyledons and cotyledonary node explants of Toona ciliata. J. For. Res. 2021, 32, 967–974. [Google Scholar] [CrossRef]
- Micheli, M.; Regni, L.; da Silva, D.F. Encapsulation in Calcium Alginate of Nodes from Stolons of Mentha spicata L. Horticulturae 2022, 8, 456. [Google Scholar] [CrossRef]
- Benelli, C.; Micheli, M.; De Carlo, A. An improved encapsulation protocol for regrowth and conservation of four ornamental species. Acta Soc. Bot. Pol. 2017, 86, 3559. [Google Scholar] [CrossRef] [Green Version]
- Kamińska, M.; Gołębiewski, M.; Tretyn, A.; Trejgell, A. Efficient long-term conservation of Taraxacum pieninicum synthetic seeds in slow growth conditions. Plant Cell Tissue Organ Cult. 2018, 132, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent Development in Micropropagation Techniques for Rare Plant Species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef]
- Faisal, M.; Anis, M. Regeneration of plants from alginate-encapsulated shoots of Tylophora indica (Burm. f.) Merrill, an endangered medicinal plant. J. Hortic. Sci. Biotechnol. 2007, 82, 351–354. [Google Scholar] [CrossRef]
- Kikowska, M.; Sliwinska, E.; Thiem, B. Micropropagation and Production of Somatic Seeds for Short-Term Storage of the Endangered Species Eryngium alpinum L. Plants 2020, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, V.; Kumar, A.; Gokul, S.; Verma, R.S.; Rahman, L.; Sundaresan, V. Micropropagation, encapsulation, and conservation of Decalepis salicifolia, a vanillin isomer containing medicinal and aromatic plant In Vitro. Cell. Dev. Biol. Plant. 2020, 56, 526–537. [Google Scholar] [CrossRef]
- Asadi, R.; Abdollahi, M.R.; Moosavi, S.S.; Mirzaie-Asl, A. Alginate encapsulation of micro-cuttings in endangered Satureja khuzistanica species: A promising method for obtaining genetically stable plants with high rosmarinic acid content. Plant Cell Tissue Organ Cult. 2022, 151, 307–320. [Google Scholar] [CrossRef]
- Paunescu, A. Biotechnology for endangered plant conservation: A critical overview. Rom. Biotechnol. Lett. 2009, 14, 4095–4103. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Shtereva, L.A.; Vassilevska-Ivanova, R.D.; Kraptchev, B.V. In vitro cultures for micropropagation, mass multiplication and preservation of an endangered medicinal plant Sideritis scardica Griseb. Bot. Serbica 2015, 39, 11–120. Available online: https://botanicaserbica.bio.bg.ac.rs/arhiva/pdf/2015_39_2_633_full.pdf (accessed on 10 October 2022).
- Dixon, R.A.; Gonzales, R.A. (Eds.) Plant Cell Culture: A Practical Approach, 2nd ed.; IRL Press: Oxford, UK, 1996; pp. 154–155. [Google Scholar]
- Krigas, N.; Mouflis, G.; Grigoriadou, K.; Maloupa, E. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in-situ collection data with plant propagation and ex situ cultivation. Biodivers. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-Facilitated Effective Propagation Protocols of the Endangered Local Endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving Ex Situ Conservation Needs and Its Future Sustainable Utilization as an Ornamental. Plants 2020, 9, 1465. [Google Scholar] [CrossRef]
- Hlatshwayo, N.A.; Amoo, S.O.; Olowoyo, J.O.; Doležal, K. Efficient Micropropagation Protocol for the Conservation of the Endangered Aloe peglerae, an Ornamental and Medicinal Species. Plants 2020, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Bertsouklis, K.; Panagaki, K.-P. In vitro Germination and Propagation of Dyckia brevifolia, An Ornamental and Endangered Bromeliad. Horticulturae 2022, 8, 390. [Google Scholar] [CrossRef]
- Paz, M.M.; Martinez, J.C.; Kalvig, A.B.; Fonger, T.M.; Wang, K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Kar, S.K.; Rout, K.K.; Barik, D.P.; Panda, P.C.; Naik, S.K. Assessment of genetic and biochemical fidelity of field established Hedychium coronarium J. Koenig regenerated from axenic cotyledonary node on meta-topolin supplemented medium. Ind. Crop Prod. 2019, 134, 206–215. [Google Scholar] [CrossRef]
- Tambarussi, E.V.; Rogalski, M.; Galeano, E.; Brondani, G.E.; Martin, V.D.; Silva, L.A.; Carrer, H. Efficient and new method for Tectona grandis in vitro regeneration. Crop Breed. Appl. Biotechnol. 2017, 17, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Meurer, C.; Dinkins, R.; Collins, G. Factors affecting soybean cotyledonary node transformation. Plant Cell Rep. 1998, 18, 180–186. [Google Scholar] [CrossRef]
- Li, Z.; Tan, X.; Liu, Z.; Lin, Q.; Zhang, L.; Yuan, J.; Zeng, Y.; Wu, L. In vitro propagation of Camellia oleifera Abel. using hypocotyl, cotyledonary node, and radicle explants. HortScience 2016, 51, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Jafari, M.; Daneshvar, M.H.; Lotfi, A. In vitro shoot proliferation of Passiflora caerulea L. via cotyledonary node and shoot tip explants. BioTechnologia 2017, 98, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Thorpem, T.A. In vitro organogenesis and somatic embryogenesis: Physiological and biochemical aspects. In Morphogenesis in Plants; Roubelakis-Angelakis, K.A., Tram Thanh Van, K., Eds.; Plenum Press: New York, NY, USA, 1993; pp. 19–38. [Google Scholar]
- Holobiuc, I.; Paunescu, A.; Blindu, R. Ex situ conservation using in vitro methods in some Caryophyllaceae plant species from the Red List of vascular plants in Romania. Rom. J. Biol. Plant Biol. 2007, 49, 3–16. [Google Scholar]
- Rafiq, S.; Wagay, N.A.; Bhat, I.A.; Kaloo, Z.A.; Rashid, S.; Lin, F.; El-Abedin, T.K.Z.; Wani, S.H.; Mahmoud, E.A.; Almutairi, K.F.; et al. In vitro propagation of Aconitum chasmanthum Stapf Ex Holmes: An endemic and critically endangered plant species of the western Himalaya. Horticulturae 2021, 7, 586. [Google Scholar] [CrossRef]
- Capuana, M.; Nissim, W.G.; Klein, J.D. Protocol for In vitro Propagation of Salix acmophylla (Boiss.). Studies on Three Ecotypes. Forests 2022, 13, 1124. [Google Scholar] [CrossRef]
- Feito, I.; Gea, M.A.; Fernández, B.; Rodríguez, R. Endogenous plant growth regulators and rooting capacity of different walnut tissues. J. Plant Growth Regul. 1996, 19, 101–108. [Google Scholar] [CrossRef]
- Choffe, K.L.; Murch, S.J.; Saxena, P.K. Regeneration of Echinacea purpurea: Induction of root organogenesis from hypocotyl and cotyledon explants. Plant Cell Tissue Organ Cult. 2000, 62, 227–234. [Google Scholar] [CrossRef]
- Erst, A.A.; Yakubov, V.V. Regenerative In Vitro Capacity of Rare Species Rhodiola rosea L. from Various Habitats. Contemp. Probl. Ecol. 2019, 12, 368–376. [Google Scholar] [CrossRef]
- Nouaim, R.; Mangin, G.; Breuil, M.C.; Chaussod, R. The argan tree (Argania spinosa) in Morocco: Propagation by seeds, cuttings and in-vitro techniques. Agrofor. Syst. 2002, 54, 71–81. [Google Scholar] [CrossRef]
- Panahandeh, J.; Farhadi, N.; Motallebi-Azar, A.R.; Alizadeh, S. Improved in vitro culture and multiplication of different Allium hirtifolium Bioss. ecotypes. Acta Hortic. 2016, 1143, 105–110. [Google Scholar] [CrossRef]
- Kumari, P.; Mehta, A. In vitro root proliferation from different explants of Solanum nigrum L. Vegetos 2022, 1–7. [Google Scholar] [CrossRef]
- He, J.; Plácido, J.P.A.; Pateraki, I.; Kampranis, S.; Favero, B.T.; Lütken, H. Hairy Root Induction of Taxus baccata L. by Natural Transformation with Rhizobium rhizogenes. Horticulturae 2023, 9, 4. [Google Scholar] [CrossRef]
- Ho, T.T.; Le, K.C.; Kim, S.W.; Park, S.Y. Culture condition optimization and FT-IR analysis of Polygonum multiflorum Thunbadventitious root cultures grown in an air-lift bioreactor system. Plant Cell Tissue Organ Cult. 2021, 144, 371–381. [Google Scholar] [CrossRef]
- Mehrotra, S.; Mishra, S.; Srivastava, V. Hairy Roots Biotechnology Unzipped: A Journey of Reality and Promises. In Hairy Root Cultures Based Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–10. [Google Scholar]
- Hussain, M.J.; Abbas, Y.; Nazli, N.; Fatima, S.; Drouet, S.; Hano, C.; Abbasi, B.H. Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. Plants 2022, 11, 439. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [Green Version]
- West, T.P.; Ravindra, M.B.; Preece, J.E. Encapsulation, cold storage, and growth of Hibiscus moscheutos nodal segments. Plant Cell Tissue Organ Cult. 2006, 87, 223–231. [Google Scholar] [CrossRef]
- Singh, S.K.; Rai, M.K.; Asthana, P.; Pandey, S.; Jaiswal, V.S.; Jaiswal, U. Plant regeneration from alginate-encapsulated shoot tips of Spilanthes acmella (L.) Murr., a medicinally important and herbal pesticidal plant species. Acta Physiol. Plant. 2009, 31, 649–653. [Google Scholar] [CrossRef]
- Kundu, S.; Salma, U.; Ali, M.N.; Mandal, N. Conservation, ex vitro direct regeneration, and genetic uniformity assessment of alginate-encapsulated nodal cuttings of Sphagneticola calendulacea (L.) Pruski. Acta Physiol. Plant. 2018, 40, 53–63. [Google Scholar] [CrossRef]
- Kulus, D. Effect of Bead Composition, PVS Type, and Recovery Medium in Cryopreservation of Bleeding Heart ‘Valentine’—Preliminary Study. Agronomy 2020, 10, 891. [Google Scholar] [CrossRef]
- Mounce, R.; Smith, P.; Brockington, S. Ex situ conservation of plant diversity in the world’s botanic gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity conservation and conservation biotechnology tools In Vitro. Cell. Dev. Biol.-Plant 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity In Vitro. Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. In vitro plant recovery from alginate encapsulated Chrysanthemum grandiflorum /Ramat./ Kitam. shoot tips. Prop. Ornam. Plants 2014, 14, 3–12. [Google Scholar]
- Hummer, K.E.; Reed, B.M. Establishment and operation of a temperate clonal field genebank. In Proceedings of the Management of Field and In Vitro Germplasm Collections: Proceedings of a Consultation Meeting, Cali, Colombia, 15–20 January 1996; Engelmann, F., Ed.; IPGRI: Rome, Italy, 2000; pp. 29–31. [Google Scholar]
- Keller, E.R.J.; Kaczmarczyk, A.; Senula, A. Cryopreservation for plant genebanks: A matter between high expectations and cautious reservation. CryoLetters 2008, 29, 53–62. [Google Scholar]







| Population | Explant | Medium | Shooting (%) | Total Shoot Number | HS † Number | Shoot Length (cm) | Node Number | MI †† | Rooting (%) | Root Number Ranking ††† | Root Length (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | CN | -(Hf) | 87.5 | 2.0 b | 0.9 bcd | 2.0 bc | 2.6 bcd | 5.8 bc | 87.5 | 1.4 bc | 1.8 cd |
| BA | 66.6 | 2.3 ab | 0.3 d | 1.3 abc | 2.3 abcd | 3.3 d | 29.2 | 1.0 bc | 0.4 d | ||
| NE | -(Hf) | 92.8 | 2.0 b | 1.7 ab | 3.4 a | 3.6 abc | 10.5 a | 85.7 | 1.3 bc | 2.8 ab | |
| BA | 92.8 | 2.8 a | 1.8 a | 1.1 c | 1.9 d | 4.7 bcd | 28.6 | 1.0 bc | 0.7 d | ||
| HY | CN | -(Hf) | 75.0 | 1.6 b | 0.6 cd | 1.6 bc | 2.6 abcd | 3.2 d | 66.7 | 1.3 bc | 1.8 bcd |
| BA | 85.0 | 1.8 b | 0.9 bcd | 1.2 c | 2.3 cd | 3.0 d | 30.0 | 1.0 bc | 0.8 d | ||
| NE | -(Hf) | 85.7 | 1.8 bc | 1.3 abc | 1.5 c | 2.7 bcd | 3.9 cd | 39.3 | 1.0 c | 0.9 d | |
| BA | 91.6 | 2.8 a | 1.5 abc | 1.5 c | 2.8 bcd | 6.4 b | 20.8 | 1.0 bc | 0.3 d | ||
| PS | CN | -(Hf) | 95.6 | 1.9 b | 1.6 abc | 3.1 ab | 3.9 ab | 9.4 a | 95.8 | 2.0 a | 3.4 a |
| BA | 95.0 | 2.0 b | 1.1 abcd | 0.9 c | 2.1 d | 2.9 d | 70.0 | 1.0 c | 0.7 d | ||
| NE | -(Hf) | 100.0 | 1.9 b | 1.6 abc | 2.9 ab | 4.5 a | 9.1 a | 96.4 | 1.6 ab | 2.4 bc | |
| BA | 100.0 | 2.4 ab | 0.9 bcd | 1.1 c | 2.0 d | 4.4 bcd | 35.7 | 1.0 bc | 0.9 d | ||
| Significance of three-way ANOVA | |||||||||||
| Population | * | *** | *** | ||||||||
| Medium | ns | *** | *** | * | |||||||
| Population × Medium | ns | ns | * | *** | *** | *** | ns | *** | * | ||
| Explant type | * | *** | *** | ns | * | ||||||
| Population × Explant type | ns | ns | *** | ns | ns | ns | ns | ns | * | ||
| Medium × Explant type | ns | * | ns | ns | ns | *** | ns | ns | ns | ||
| Population × Explant type × Medium | ns | ns | ns | ns | ns | *** | ns | ns | * | ||
| Mean values of factors †††† | |||||||||||
| Shooting (%) | Rooting (%) | ||||||||||
| Population | Mean | Explant | Mean | Population | Mean | Explant | Mean | Medium | Mean | ||
| PS | 97.6 a | NE | 93.9 a | PS | 74.0 a | CN | 63.7 a | 0.0 | 79.2 a | ||
| PR | 85.0 b | CN | 84.4 b | PR | 57.7 b | NE | 51.8 a | 0.5 | 34.7 b | ||
| HY | 85.0 b | HY | 35.7 c | ||||||||
| Population | BA/NAA (mg L−1) | Shooting (%) | Total Shoot Number | HS † Number | Shoot Length (cm) | Node Number | MI †† | Rooting (%) | Root Number Ranking ††† | Root Length (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| PR | 0/0 | 78.0 | 1.4 | 1.4 | 5.3 a | 4.0 | 9.7 ab | 78.0 | 2.6 a | 4.0 a |
| 0.5/0 | 81.5 | 3.3 | 1.5 | 2.1 bcd | 1.9 | 9.4 ab | 46.9 | 1.0 c | 0.9 c | |
| 0.5/0.1 | 69.0 | 2.4 | 1.5 | 2.0 bcd | 2.0 | 5.5 cde | 34.3 | 1.0 c | 0.7 c | |
| 1.0/0 | 72.0 | 2.8 | 1.1 | 1.8 bcd | 2.0 | 6.0 bcd | 6.3 | 1.0 c | 2.0 bc | |
| 1.0/0.1 | 84.0 | 3.4 | 0.9 | 2.3 bcd | 3.1 | 10.9 a | 31.3 | 1.0 c | 1.0 c | |
| HY | 0/0 | 80.0 | 1.6 | 0.9 | 3.1 b | 3.7 | 6.6 bcd | 50.0 | 1.0c | 0.4 c |
| 0.5/0 | 70.0 | 1.8 | 0.6 | 0.9 d | 1.9 | 2.0 e | 0.0 | 0.0 d | 0.0 d | |
| 0.5/0.1 | 62.5 | 1.8 | 0.8 | 1.3 cd | 2.5 | 2.0 e | 4.0 | 1.5 bc †††† | 2.7 b †† | |
| 1.0/0 | 55.0 | 2.4 | 1.1 | 1.6 bcd | 1.9 | 3.5 de | 0.0 | 0.0 d | 0.0 d | |
| 1.0/0.1 | 50.0 | 2.4 | 0.3 | 1.7 bcd | 1.9 | 4.3 cde | 0.0 | 0.0 d | 0.0 d | |
| PS | 0/0 | 85.0 | 2.1 | 0.9 | 2.5 bc | 3.7 | 7.4 bcd | 65.0 | 2.0 b | 2.9 b |
| 0.5/0 | 90.0 | 2.8 | 0.8 | 1.2 d | 1.9 | 4.9 cde | 20.0 | 1.0 c | 0.9 c | |
| 0.5/0.1 | 90.0 | 3.1 | 0.7 | 1.4 cd | 2.3 | 6.3 bcd | 37.5 | 1.2 c | 0.9 c | |
| 1.0/0 | 87.5 | 3.6 | 0.6 | 1.4 cd | 1.8 | 7.5 abc | 25.0 | 1.1 c | 0.8 c | |
| 1.0/0.1 | 87.5 | 3.6 | 0.5 | 1.3 cd | 1.7 | 7.0 bcd | 37.5 | 1.2 c | 0.9 c | |
| Significance of two-way ANOVA | ||||||||||
| Population | *** | *** | *** | *** | ns | *** | *** | - | - | |
| BA/NAA | ns | *** | *** | *** | *** | *** | *** | - | - | |
| Population × BA/NAA | ns | ns | ns | *** | ns | *** | ns | - | - | |
| Mean values of factors ††††† | ||||||||||
| Shooting (%) | Total shoot number | Rooting (%) | ||||||||
| Population | Mean | Population | Mean | BA/NAA | Mean | Population | Mean | BA/NAA | Mean | |
| PS | 88.0 a | PS | 3.1 a | 0/0 | 1.7 b | PS | 37.0 a | 0/0 | 64.4 a | |
| PR | 76.9 ab | PR | 2.7 a | 0.5/0.1 | 2.4 ab | PR | 39.0 a | 0.5/0.1 | 25.3 b | |
| HY | 63.5 b | HY | 2.0 b | 1.0/0.1 | 3.1 a | HY | 10.2 b | 1.0/0.1 | 22.9 b | |
| 0.5/0 | 2.6 a | 0.5/0 | 22.3 b | |||||||
| 1.0/0 | 2.9 a | 1.0/0 | 10.4 b | |||||||
| High shoot number | Node number | |||||||||
| Population | Mean | BA/NAA | Mean | BA/NAA | Mean | |||||
| PS | 0.7 b | 0/0 | 1.1 a | 0/0 | 3.8 a | |||||
| PR | 1.3 a | 0.5/0.1 | 1.0 a | 0.5/0.1 | 2.2 b | |||||
| HY | 0.8 b | 1.0/0.1 | 0.6 b | 1.0/0.1 | 2.2 b | |||||
| 0.5/0 | 1.0 a | 0.5/0 | 1.9 b | |||||||
| 1.0/0 | 1.0 ab | 1.0/0 | 1.9 b | |||||||
| Principal Components (PC) | ||
|---|---|---|
| PC1 | PC2 | PC3 |
| % Contribution of variability | ||
| 54.0 | 20.1 | 12.4 |
| Related variables | ||
| SL | SH | HS |
| NO | TS | |
| RO | MI | |
| RN | ||
| RL | ||
| Treatment | Growth (Days) | Shooting (%) | Total Shoot Number | HS † Number | Shoot Length (cm) | Node Number | MI †† | Rooting (%) | Root Number Ranking ††† | Root Length (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| NEE | 30 | 84.0 | 2.0 c | 1.1 b | 2.5 | 2.7 b | 2.8 | 68.0 | 1.2 | 1.2 |
| E0d | 58.0 | 2.0 c | 1.1 b | 2.4 | 2.3 b | 1.9 | 33.0 | 1.9 | 1.9 | |
| E30d | 78.0 | 1.9 c | 1.1 b | 2.1 | 2.3 b | 2.5 | 42.0 | 1.9 | 1.9 | |
| E60d | 75.0 | 2.3 bc | 1.2 b | 2.6 | 2.9 b | 2.9 | 55.0 | 1.6 | 1.6 | |
| NEE | 50 | 84.0 | 2.0 c | 1.2 b | 2.5 | 2.7 b | 2.8 | 72.0 | 1.4 | 1.4 |
| E0d | 58.0 | 2.3 bc | 1.5 ab | 3.4 | 3.4 ab | 2.2 | 39.0 | 2.4 | 2.4 | |
| E30d | 72.0 | 3.4 ab | 2.1 a | 3.3 | 3.4 ab | 4.1 | 45.0 | 2.2 | 2.2 | |
| E60d | 72.0 | 3.8 a | 1.2 b | 4.3 | 4.1 a | 4.6 | 70.0 | 2.3 | 2.3 | |
| Significance of two-way ANOVA | ||||||||||
| Treatment | ns | *** | ** | *** | *** | *** | ||||
| Growth | ns | *** | ** | ns | *** | *** | ||||
| Treatment × Growth | ns | *** | *** | ns | *** | ns | ns | ns | ns | |
| Mean values of factors †††† | ||||||||||
| Shooting (%) | Shoot length (cm) | |||||||||
| Treatment | Mean | Growth | Mean | Treatment | Mean | Growth | Mean | |||
| NEE | 87.0 a | 30 days | 72.5 a | NEE | 2.5 b | 30 days | 2.4 b | |||
| E0d | 58.0 a | 50 days | 70.0 a | E0d | 2.9 ab | 50 days | 3.4 a | |||
| E30d | 75.0 a | E30d | 2.7 ab | |||||||
| E60d | 73.5 a | E60d | 3.4 a | |||||||
| MI | Root number ranking | |||||||||
| Treatment | Mean | Growth | Mean | Treatment | Mean | Growth | Mean | |||
| NEE | 2.8 ab | 30 days | 2.5 b | NEE | 1.3 b | 30 days | 1.6 b | |||
| E0d | 2.1 b | 50 days | 3.4 a | E0d | 2.2 a | 50 days | 2.1 a | |||
| E30d | 3.3 a | E30d | ||||||||
| E60d | 3.8 a | E60d | ||||||||
| Rooting (%) | Root length (cm) | |||||||||
| Treatment | Mean | Growth | Mean | Treatment | Mean | Growth | Mean | |||
| NEE | 72.0 a | 30 days | 50.5 a | NEE | 2.1 b | 30 days | 2.3 b | |||
| E0d | 36.0 b | 50 days | 56.0 a | E0d | 3.9 a | 50 days | 2.7 a | |||
| E30d | 43.0 b | E30d | 2.3 b | |||||||
| E60d | 62.5 a | E60d | 2.5 b | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertsouklis, K.; Tsopela, S. In Vitro Propagation of Three Populations of the Endangered, Greek Endemic Cerastium candidissimum and Short-Term Storability of Alginate-Encapsulated Shoot Explants for Exploitation and Conservation. Horticulturae 2023, 9, 273. https://doi.org/10.3390/horticulturae9020273
Bertsouklis K, Tsopela S. In Vitro Propagation of Three Populations of the Endangered, Greek Endemic Cerastium candidissimum and Short-Term Storability of Alginate-Encapsulated Shoot Explants for Exploitation and Conservation. Horticulturae. 2023; 9(2):273. https://doi.org/10.3390/horticulturae9020273
Chicago/Turabian StyleBertsouklis, Konstantinos, and Stella Tsopela. 2023. "In Vitro Propagation of Three Populations of the Endangered, Greek Endemic Cerastium candidissimum and Short-Term Storability of Alginate-Encapsulated Shoot Explants for Exploitation and Conservation" Horticulturae 9, no. 2: 273. https://doi.org/10.3390/horticulturae9020273