Alteration of Flower Yield and Phytochemical Compounds of Saffron (Crocus sativus L.) by Application of Different Light Qualities and Growth Regulators
Abstract
:1. Introduction
2. Materials and Methods
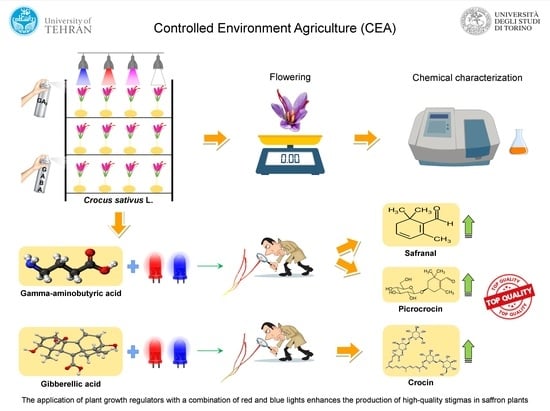
2.1. Plant Materials and Growth Conditions
2.2. Determining the Yield of Saffron Flower
2.3. Determination of Phytochemical Compounds
2.4. Statistical Analysis
3. Results
3.1. The Floral Yield of Saffron Plants
3.2. The Phytochemicals Content
3.3. Principal Component Analysis (PCA) of Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gernot Katzer’s Spice Pages. Available online: http://gernot-katzers-spice-pages.com/engl/Croc_sat.html (accessed on 23 October 2022).
- McGee, H. On Food and Cooking: The Science and Lore of the Kitchen, 2nd ed.; Scribner: New York, NY, USA, 2004; p. 423. [Google Scholar]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Gresta, F.; Lombardo, G.M.; Siracusa, L.; Ruberto, G. Saffron, an alternative crop for sustainable agricultural systems. A review. Agron. Sustain. Dev. 2008, 28, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, R.; Koocheki, A. Sustainable cultivation of saffron in Iran. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 25, pp. 170–171. [Google Scholar]
- Business Insider. Available online: https://www.businessinsider.com/why-saffron-world-most-expensive-spice-2018-4 (accessed on 29 October 2022).
- BBC News. Available online: https://www.bbc.com/news/business-41110151 (accessed on 23 October 2022).
- Kothari, D.; Thakur, R.; Kumar, R. Saffron (crocus sativus L.): Gold of the spices-a comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677. [Google Scholar] [CrossRef]
- Good Practices for Saffron Production in Kashmir. Available online: https://diragrijmu.nic.in/saffron/saffron-manual.pdf (accessed on 9 January 2023).
- Ting, K.C.; Lin, T.; Davidson, P.C. Integrated urban controlled environment agriculture systems. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E.S., Eds.; Springer: Singapore, 2016; pp. 19–36. [Google Scholar]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue light improves photosynthetic performance and biomass partitioning toward harvestable organs in saffron (Crocus sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Controlled Environment Agriculture Center. The University of Arizona. Available online: https://ceac.arizona.edu/about/about-us (accessed on 29 October 2022).
- Birkby, J. Vertical Farming. ATTRA Sustain. Agric. 2016. Available online: https://attra.ncat.org/publication/Vertical-Farming/ (accessed on 16 January 2022).
- Tunio, M.H.; Gao, J.M.; Qureshi, W.A.; Sheikh, S.A.; Chen, J.D.; Chandio, F.A.; Lakhiar, I.A.; Solangi, K.A. Effects of droplet size and spray interval on root-to-shoot ratio, photosynthesis efficiency, and nutritional quality of aeroponically grown butterhead lettuce. Int. J. Agric. Biol. Eng. 2022, 15, 79–88. [Google Scholar] [CrossRef]
- Uddin, M.R.; Suliaman, M.F. Energy Efficient Smart Indoor Fogponics Farming System. In Proceedings of the 3rd International Conference on Smart City Innovation, Bali, Indonesia, 5–6 August 2020; IOP Conference Series: Earth and Environmental Science. Volume 673, p. 012012. [Google Scholar]
- Aghhavani-Shajari, M.; Fallahi, H.R.; Sahabi, H.; Kaveh, H.; Branca, F. Production systems and methods affect the quality and the quantity of saffron (Crocus sativus L.). Span. J. Agric. Res. 2021, 19, 14. [Google Scholar] [CrossRef]
- Zhou, T.; Qiu, X.; Zhao, L.; Yang, W.; Wen, F.; Wu, Q.; Yan, J.; Xu, B.; Chen, J.; Ma, Y.; et al. Optimal light intensity and quality increased the saffron daughter corm yield by inhibiting the degradation of reserves in mother corms during the reproductive stage. Ind. Crops Prod. 2022, 176, 114396. [Google Scholar] [CrossRef]
- Jung, W.S.; Chung, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. Application of light-emitting diodes for improving the nutritional quality and bioactive compound levels of some crops and medicinal plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Academic Press: London, UK, 2020; pp. 7–33. [Google Scholar]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Daylami, S.D.; Serek, M.; Woltering, E.; Li, T. Blue light improves vase life of carnation cut flowers through its effect on the antioxidant defense system. Front. Plant Sci. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Kafi, M.; Kalate-Jari, S.; Matinizadeh, M. Tulip response to different light sources. Anim. Plant Sci. 2018, 28, 539–545. [Google Scholar]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of growth, water use efficiency, chlorophyll fluorescence, and stomatal characteristics of lettuce plants to light intensity. Plant Growth Regul. 2020, 40, 2191–2207. [Google Scholar] [CrossRef]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Rasul, F.; Li, W.; Tian, H.; Mo, Z.; Duan, M.; Tang, X. Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice 2013, 6, 9. [Google Scholar] [CrossRef]
- Martinez, M.E.; Jorquera, L.; Poirrier, P.; Díaz, K.; Chamy, R. Effect of the carbon source and plant growth regulators (PGRs) in the induction and maintenance of an in vitro callus culture of Taraxacum officinale (L.) weber Ex F.H. Wigg. Agronomy 2021, 11, 1181. [Google Scholar] [CrossRef]
- Prins, C.L.; Vieira, I.J.C.; Freitas, S.P. Growth regulators and essential oil production. Braz. J. Plant Physiol. 2010, 22, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V.; Lastochkina, O. Diverse role of γ-aminobutyric acid in dynamic plant cell responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Roberts, M.R. Does GABA act as a signal in plants? Hints from molecular studies. Plant Signal Behav. 2007, 2, 408–409. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.L.L.; Rodrigues, C.; Costa, J.D.L.; Machado, M.P.; Penha, R.D.O.; Biasi, L.A.; Vandenberghe, L.P.D.S.; Soccol, C.R. Gibberellic acid fermented extract obtained by solid-state fermentation using citric pulp by Fusarium moniliforme: Influence on Lavandula angustifolia Mill. cultivated in vitro. Pak. J. Bot. 2013, 45, 2057–2064. [Google Scholar]
- Camara, M.C.; Rodrigues, C.; da Silva, A.L.L.; Vandenberghe, L.; Soccol, C. General aspects and applications of gibberelins and gibberellic acid in plants. In Gibberellins and Gibberellic Acid: Biosynthesis, Regulation and Physiological Effects, 1st ed.; Hardy, J., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 1–21. [Google Scholar]
- Keshtkar, H.R.; Azarnivand, H.; Etemad, V.; Moosavi, S.S. Seed dormancy-breaking and germination requirements of Ferula ovina and Ferula gummosa. Desert 2008, 13, 45–51. [Google Scholar]
- Riley, J.M. Gibberellic acid for fruit set and seed germination. CRFG 1987, 19, 10–12. [Google Scholar]
- Edwards, M. Dormancy in seeds of charlock (Sinapis arvensis L.): Early effects of gibberellic acid on the synthesis of amino acids and proteins. Plant Physiol. 1976, 58, 626–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrungoo, N.K.; Farooq, S. Influence of GA3 and NAA on certain carbohydrate fractions in corms of saffron crocus (Crocus sativus L.) during development. Acta Soc. Bot. Pol. 1989, 58, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Braidot, E.; Petrussa, E.; Peresson, C.; Patui, S.; Bertolini, A.; Tubaro, F.; Wählby, U.; Coan, M.; Vianello, A.; Zancani, M. Low-intensity light cycles improve the quality of lamb’s lettuce (Valerianella olitoria L. Pollich) during storage at low temperature. Postharvest Biol. Technol. 2014, 90, 15–23. [Google Scholar] [CrossRef]
- Chen, X.; Guo, W.; Xue, X.; Wang, L.; Qiao, X. Growth and quality responses of ‘Green Oak Leaf’ lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Chung, I.M.; Paudel, N.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. The influence of light wavelength on growth and antioxidant capacity in Pachyrhizus erosus (L.) Urban. Plant Growth Regul. 2020, 39, 296–312. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting broccoli accumulate higher concentrations of nutritionally important metabolites under narrow-band light-emitting diode lighting. Amer. Soc. Hort. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Brazaitytė, A.; Jankauskienė, J.; Viršilė, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Sirtautas, R.; Viršilė, A.; Sakalauskaitė, J.; Sakalauskienė, S.; Duchovskis, P. LED illumination affects bioactive compounds in romaine baby leaf lettuce. Sci. Food Agric. 2013, 93, 3286–3291. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Nebauer, S.G.; Sánchez, M.; Molina, R.V. Effect of corm size, water stress and cultivation conditions on photosynthesis and biomass partitioning during the vegetative growth of saffron (Crocus sativus L.). Ind. Crops Prod. 2012, 39, 40–46. [Google Scholar] [CrossRef]
- Molina, R.V.; Valero, M.; Navarro, Y.; Guardiola, J.L.; García-Luis, A. Temperature effects on flower formation in saffron (Crocus sativus L.). Sci. Hortic. 2005, 103, 361–379. [Google Scholar] [CrossRef]
- Molina, R.V.; Valero, M.; Navarro, Y.; García-Luis, A.; Guardiola, J.L. Low temperature storage of corms extends the flowering season of saffron (Crocus sativus L.). Hortic. Sci. Biotechnol. 2005, 80, 319–326. [Google Scholar] [CrossRef]
- Kalhor, M.S.; Aliniaeifrad, S.; Seif, M.; Asayesh, E.J.; Bernard, F.; Hassani, B.; Li, T. Enhanced salt tolerance and photosynthetic performance: Implication of γ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018, 130, 157–172. [Google Scholar] [CrossRef]
- Mzabri, I.; Rimani, M.; Kouddane, N.; Berrichi, A. Study of the effect of pretreatment of corms by different concentrations of gibberellic acid and at different periods on the growth, flowering, and quality of saffron in eastern Morocco. Adv. Agric. 2021, 2021, 9979827. [Google Scholar] [CrossRef]
- Fallahi, H.R.; Abbasi Aval Bohlooli, S.; Pahlavan, Z.; Hosseini, S.M.; Hosseini, S.A.H.; Ghohestani-Bojd, P. Saffron vegetative growth as affected by transplanting and direct corm planting under field conditions. J. Hortic. Postharvest Res. 2021, 4, 1–10. [Google Scholar] [CrossRef]
- ISO 3632-2:2010(en); Spices—Saffron (Crocus sativus L.)—Part 2: Test methods. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/obp/ui/#iso:std:iso:3632:-2:ed-2:v1:en (accessed on 29 October 2022).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org (accessed on 29 October 2022).
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer-Verlag Press: New York, NY, USA, 1986. [Google Scholar]
- Lage, M.; Cantrell, C.L. Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci. Hortic. 2009, 121, 366–373. [Google Scholar] [CrossRef]
- Esmaeili, S.; Aliniaeifard, S.; Dianati Daylami, S.; Karimi, S.; Shomali, A.; Didaran, F.; Telesiński, A.; Sierka, E.; Kalaji, H.M. Elevated light intensity compensates for nitrogen deficiency during chrysanthemum growth by improving water and nitrogen use efficiency. Sci. Rep. 2022, 12, 10002. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.T. Distinct responses to light in plants. Plants 2020, 9, 894. [Google Scholar] [CrossRef]
- Parks, B.M. The red side of photomorphogenesis. Plant Physiol. 2003, 133, 1437–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalighi, S.; Zare Mehrjerdi, M.; Aliniaefard, S. Light quality affects phytochemicals content and antioxidant capacity of Satureja hortensis. South-West. J. Hortic. Biol. Environ. 2021, 12, 99–117. [Google Scholar]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Meshram, J.H.; Singh, S.B.; Raghavendra, K.P.; Waghmare, V.N. Drought stress tolerance in cotton: Progress and perspectives. In Climate Change and Crop Stress: Molecules to Ecosystems, 1st ed.; Shanker, A.K., Shanker, C., Anand, A., Maheswari, M., Eds.; Academic Press: London, UK, 2022; pp. 135–169. [Google Scholar]
- Patel, A.; Tiwari, S.; Parihar, P.; Singh, R.; Prasad, S.M. Carbon nanotubes as plant growth regulators: Impacts on growth, reproductive system, and soil microbial community. In Nanomaterials in Plants, Algae and Microorganisms: Concepts and Controversies, 1st ed.; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: London, UK, 2019; Volume 2, pp. 23–42. [Google Scholar]
- Sabagh, A.E.; Mbarki, S.; Hossain, A.; Iqbal, M.A.; Islam, M.S.; Raza, A.; Llanes, A.; Reginato, M.; Rahman, M.A.; Mahboob, W.; et al. Potential role of plant growth regulators in administering crucial processes against abiotic stresses. Front. Agron. 2021, 3, 648694. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Fiebig, A.; Noga, G.; Hunsche, M. Influence of light quality on leaf physiology of sweet pepper plants grown under drought. Theor. Exp. Plant Physiol. 2018, 30, 287–296. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, N.; Uno, Y.; Kuroki, S.; Miyagawa, S.; Yamashita, Y.; Hamaguchi, Y.; Ueda, Y.; Kobayashi, M.; Kaji, K.; Itoh, H. Effect of far-red light on saffron (crocus sativus L.) growth and crocin yields. Environ. Control Biol. 2018, 56, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Sng, B.J.R.; Mun, B.; Mohanty, B.; Kim, M.; Phua, Z.W.; Yang, H.; Lee, D.Y.; Jang, I.C. Combination of red and blue light induces anthocyanin and other secondary metabolite biosynthesis pathways in an age-dependent manner in Batavia lettuce. Plant Sci. 2021, 310, 110977. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Ashraf, U.; Li, G.; Li, Y.; Lu, W.; Gao, L.; Han, F.; Hu, J. Exogenous γ-aminobutyric acid (GABA) application improved early growth, net photosynthesis, and associated physio-biochemical events in maize. Front. Plant Sci. 2016, 7, 919. [Google Scholar] [CrossRef] [Green Version]
- Fait, A.; Nesi, A.N.; Angelovici, R.; Lehmann, M.; Pham, P.A.; Song, L.; Haslam, R.P.; Napier, J.A.; Galili, G.; Fernie, A.R. Targeted enhancement of glutamate-to-γ-aminobutyrate conversion in Arabidopsis seeds affects carbon-nitrogen balance and storage reserves in a development-dependent manner. Plant Physiol. 2011, 157, 1026–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suo-ling, S.; Jing-rui, L.; Hong-bo, G.; Qing-yun, L.; Li-wen, Y.; Rui-juan, G. Effects of exogenous γ-aminobutyric acid on inorganic nitrogen metabolism and mineral elements contents of melon seedling under hypoxia stress. Acta Hortic. 2012, 4, 012. [Google Scholar]
- Jin, H.; Dilworth, M.; Glenn, A. 4-Aminobutyrate is not available to bacteroids of cowpea rhizobium MNF2030 in snake bean nodules. Arch. Microbiol. 1990, 153, 455–462. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Puthur, J.T. γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2016, 78, 57–67. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Killiny, N. Application of gamma-aminobutyric acid increased the level of phytohormones in Citrus sinensis. Planta 2018, 248, 909–918. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Kaur, R.; Zhawar, V.K. Regulation of secondary antioxidants and carbohydrates by gamma-aminobutyric acid under salinity-alkalinity stress in rice (Oryza sativa L.). Biologia Futura 2021, 72, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Silva, J.A.T. The cut flower: Postharvest considerations. J. Biol. Sci. 2003, 3, 406–442. [Google Scholar] [CrossRef] [Green Version]
- Javid, M.G.; Hoseinifard, M.S.; Allahdadi, I.; Soltani, E. Hormonal priming with BAP and GA3 induces improving yield and quality of saffron flower through promotion of carbohydrate accumulation in corm. Plant Growth Regul. 2022, 41, 205–215. [Google Scholar] [CrossRef]
- Mortazavi, H.; Asil, M.H. Effects of temperature and gibberellic acid on forcing and quality improvement of Iris (Iris hollandica cv. ‘Blue Magic’) cut flowers. Agric. Sci. Sustain. Prod. 2010, 20, 1–14. [Google Scholar]
- Mollafilabi, A. Effect of New Cropping Technologies on Growth Characteristics, Yield, Yield Components of Flower and Corm Criteria of Saffron (Crocus sativus L.). Ph. D. Thesis, Ferdowsi University of Mashhad, Mashhad, Iran, 2014. (In Persian with English summary). [Google Scholar]




| S.O.V. | df | MS | ||||
|---|---|---|---|---|---|---|
| Number of Flowers per Corm | Flower Fresh Weight (mg per Corm) | Flower Dry Weight (mg per Corm) | Stigma Fresh Weight (mg per Corm) | Stigma Dry Weight (mg per Corm) | ||
| R | 2 | 0.054 ns | 225,864.111 ** | 7255.083 ** | 727.083 ** | 9.631 * |
| PGR | 2 | 2.629 ** | 212,712.694 ** | 6869.083 ** | 1787.25 ** | 134.156 ** |
| Light quality | 3 | 0.733 ** | 54,509.435 ** | 1801.657 ** | 586.25 ** | 75.117 ** |
| PGR×light quality | 6 | 0.175 * | 5998.102 ** | 201.38 ** | 119.25 * | 17.839 ** |
| E | 22 | 0.059 | 711.626 | 23.508 | 45.265 | 1.762 |
| CV (%) | − | 21 | 6.7 | 6.7 | 19.1 | 17.6 |
| S.O.V. | df | MS | ||
|---|---|---|---|---|
| Crocin (% DW) | Picrocrocin (% DW) | Safranal (% DW) | ||
| R | 2 | 84.011 ** | 13.792 ** | 39.531 ** |
| PGR | 2 | 128.661 ** | 10.262 ** | 3.491 ** |
| Light quality | 3 | 64.67 ** | 5.599 ** | 3.489 ** |
| PGR×light quality | 6 | 6.197 ** | 1.86 ** | 0.445 ** |
| E | 22 | 0.216 | 0.02 | 0.052 |
| CV (%) | − | 2.62 | 1.1 | 6.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eftekhari, M.; Javid, M.G.; Aliniaeifard, S.; Nicola, S. Alteration of Flower Yield and Phytochemical Compounds of Saffron (Crocus sativus L.) by Application of Different Light Qualities and Growth Regulators. Horticulturae 2023, 9, 169. https://doi.org/10.3390/horticulturae9020169
Eftekhari M, Javid MG, Aliniaeifard S, Nicola S. Alteration of Flower Yield and Phytochemical Compounds of Saffron (Crocus sativus L.) by Application of Different Light Qualities and Growth Regulators. Horticulturae. 2023; 9(2):169. https://doi.org/10.3390/horticulturae9020169
Chicago/Turabian StyleEftekhari, Mostafa, Majid Ghorbani Javid, Sasan Aliniaeifard, and Silvana Nicola. 2023. "Alteration of Flower Yield and Phytochemical Compounds of Saffron (Crocus sativus L.) by Application of Different Light Qualities and Growth Regulators" Horticulturae 9, no. 2: 169. https://doi.org/10.3390/horticulturae9020169