Chemical Composition and Cytoprotective Activities of Methanolic Extract of Asplenium adiantum-nigrum L. (Aspleniaceae)
Abstract
:1. Introduction
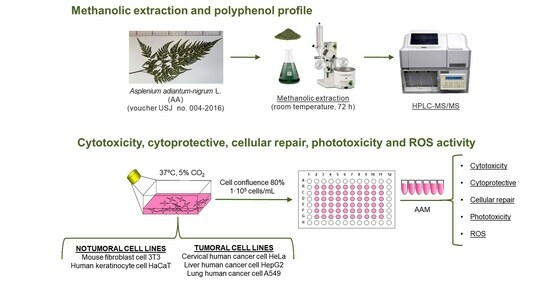
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds
2.4. Polyphenol Characterization of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS)
2.5. Cell Culture and Cytotoxicity Studies
2.6. Cytoprotective and Cellular Repair Activity of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds in Non-Tumoral Cell Lines
2.7. Phototoxicity Activity of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds in 3T3 and HaCaT Cell Lines
2.8. Intracellular Reactive Oxygen Species (ROS) Induced by H2O2 of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds in 3T3 and HaCaT Cell Lines
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Characterization of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS)
3.2. In Vitro Cell Assays
3.2.1. Cytotoxicity of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds in Non-Tumoral and Tumoral Cell Lines
3.2.2. Cytoprotective Activity of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds in 3T3 and HaCaT Cell Lines
3.2.3. Cellular Repair Activity of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds in 3T3 Tissue Cell Line
3.2.4. Phototoxicity Activity of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds in 3T3 and HaCaT Tissue Cell Lines
3.2.5. Intracellular ROS Induced by H2O2 of Methanolic Extract of Asplenium adiantum-nigrum L. Fronds in 3T3 and HaCaT Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3T3 = NIH 3T3 | NIH 3T3 mouse fibroblast cell line |
| A549 | Human Caucasian lung carcinoma |
| AAM | Methanolic extract of Asplenium adiantum-nigrum L. fronds |
| CO2 | Carbon dioxide |
| CPZ | Chlorpromazine hydrochloride |
| CV | Cell Viability |
| E | Ultraviolet dose |
| DCF | 2,7-dichlorodihydrofluorescein diacetate |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl sulfoxide |
| ECACC | European Collection of Authenticated Cell Cultures |
| EDTA | Ethylenediaminetetraacetic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FBS | Fetal Bovine Serum |
| FI | Fluorescence Intensity |
| HaCaT | Spontaneously immortalized human keratinocyte cell line |
| HeLa | Human cervix epitheloid carcinoma |
| HepG2 | Human Caucasian hepatocyte carcinoma |
| HPLC-MS/MS | High performance liquid chromatography-tandem mass spectrometry |
| H2O2 | Hydrogen peroxide |
| MTT | 2,5-diphenyl-3-(4,5-dimethyl-2-thiazolyl) tetrazolium bromide |
| NR | Neutral Red |
| NRU | Neutral Red Uptake |
| OECD | Organisation for Economic Cooperation and Development |
| ORAC | Oxygen Radical Absorbance Capacity |
| PBS | Phosphate Buffered Saline |
| PLE | Aqueous extract of Polypodium leucotomos fronds |
| PVM | Methanolic extract of Polypodium vulgare L. fronds |
| ROS | Reactive Oxygen Species |
| UV | Ultraviolet |
| UVA | Ultraviolet A |
| WHO | World Health Organization |
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 41. [Google Scholar] [CrossRef]
- Fatima, N.; Baqri, S.S.R.; Alsulimani, A.; Fagoonee, S.; Slama, P.; Kesari, K.K.; Roychoudhury, S.; Haque, S. Phytochemicals from Indian Ethnomedicines: Promising Prospects for the Management of Oxidative Stress and Cancer. Antioxidants 2021, 10, 28. [Google Scholar] [CrossRef]
- Reinaldo, R.; Santiago, A.C.P.; Medeiros, P.M.; Albuquerque, U.P. Do ferns and lycophytes function as medicinal plants? A study of their low representation in traditional pharmacopoeias. J. Ethnopharmacol. 2015, 175, 39–47. [Google Scholar] [CrossRef]
- Upreti, K.; Jalal, J.S.; Tewari, L.M.; Joshi, G.C.; Pangtey, Y.P.S.; Tewari, G. Ethnomedicinal uses of Pteridophytes of Kumaun Himalaya, Uttarakhand, India. J. Am. Sci. 2009, 5, 4. [Google Scholar]
- Singh, S.; Kharkwal, K.; Kant, R.; Sinha, B.K.; Ambrish, K. Studies on Ethnobotanical aspects of Pteridophytes in Great Himalayan National Park, Kullu, Himachal Pradesh. Phytotaxonomy 2017, 17, 43–49. [Google Scholar]
- Chang, H.-C.; Huang, G.-J.; Agrawal, D.C.; Kuo, C.-L.; Wu, C.-R.; Tsay, H.-S. Antioxidant activities and polyphenol contents of six folk medicinal ferns used as “Gusuibu”. Bot. Stud. 2007, 48, 397–406. [Google Scholar]
- Abbasi, A.M.; Khan, M.A.; Ahmad, M.; Zafar, M.; Jahan, S.; Sultana, S. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. J. Ethnopharmacol. 2010, 128, 322–335. [Google Scholar] [CrossRef]
- González, S.; Gilaberte, Y.; Philips, N. Mechanistic insights in the use of a Polypodium leucotomos extract as an oral and topical photoprotective agent. Photochem. Photobiol. Sci. 2010, 9, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Mascaraque, M.; Gilaberte, Y.; Juarranz, A.; Gonzalez, S. Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. Int. J. Mol. Sci. 2016, 17, 1026. [Google Scholar] [CrossRef] [PubMed]
- Langhansova, L.; Pumprova, K.; Haisel, D.; Ekrt, L.; Pavicic, A.; Zajickova, M.; Vanek, T.; Dvorakova, M. European ferns as rich sources of antioxidants in the human diet. Food Chem. 2021, 356, 129637. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Pumprova, K.; Antoninova, Z.; Rezek, J.; Haisel, D.; Ekrt, L.; Vanek, T.; Langhansova, L. Nutritional and Antioxidant Potential of Fiddleheads from European Ferns. Foods 2021, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Moreno Saiz, J.C.; Pataro, L.; Pajaron Sotomayor, S. Atlas of the pteridophytes of the Iberian Peninsula and the Balearic Islands. Acta Bot. Malacit. 2015, 40, 5–55. [Google Scholar]
- Akerreta, S.; Calvo, M.I.; Cavero, R.Y. Ethnoveterinary knowledge in Navarra (Iberian Peninsula). J. Ethnopharmacol. 2010, 130, 369–378. [Google Scholar] [CrossRef]
- Bonet, M.À.; Vallès, J. Ethnobotany of Montseny biosphere reserve (Catalonia, Iberian Peninsula): Plants used in veterinary medicine. J. Ethnopharmacol. 2007, 110, 130–147. [Google Scholar] [CrossRef]
- Bonet, M.À.; Agelet, A.; Vallès, J.; Villar Pérez, L. Contribution à la connaissance ethnobotanique des ptéridophytes dans les Pyrénées. Bocconea 2001, 13, 605–612. [Google Scholar]
- TheWorldFloraOnline. Asplenium adiantum-nigrum L. Available online: http://www.worldfloraonline.org/taxon/wfo-0001109303 (accessed on 13 June 2022).
- Smith, A.R.; Pryer, K.M.; Schuettpelz, E.; Korall, P.; Schneider, H.; Wolf, P.G. A classification for extant ferns. Taxon 2006, 55, 705–731. [Google Scholar] [CrossRef]
- Bonet Galobart, M.À.; Vallès Xirau, J. Etnobotànica del Montseny: Valoració i comparació d’alguns aspectes. In Plantes, Remeis i Cultura Popular Del Montseny-Etnobotànica d’una Reserva de la Biosfera; Brau, Ed.; Brau: Figueres, Spain, 2006; pp. 651–722. [Google Scholar]
- Corsi, G.; Gaspari, G.; Pagni, A.M. L’uso delle piante nell’economia domestica della Versilia collinare e montana. Atti Della Soc. Toscana Sci. Nat. Mem. Ser. B 1980, 87, 309–386. [Google Scholar]
- Zeng, W.W.; Lai, L.S. Multiple-physiological benefits of bird’s nest fern (Asplenium australasicum) frond extract for dermatological applications. Nat. Prod. Res. 2017, 33, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Font Castell, X.; de Càceres, M.; Quadrada, R.; Moreno, J.; Martí, D. Banco de Datos de Biodiversidad de Cataluña. 2015. Available online: http://biodiver.bio.ub.es/biocat/index.jsp (accessed on 25 May 2017).
- Farràs, A.; Cásedas, G.; Les, F.; Terrado, E.M.; Mitjans, M.; López, V. Evaluation of Anti-Tyrosinase and Antioxidant Properties of Four Fern Species for Potential Cosmetic Applications. Forests 2019, 10, 14. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.B.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 11. [Google Scholar] [CrossRef] [PubMed]
- Farràs, A.; Mitjans, M.; Maggi, F.; Caprioli, G.; Vinardell, M.P.; López, V. Polypodium vulgare L. (Polypodiaceae) as a Source of Bioactive Compounds: Polyphenolic Profile, Cytotoxicity and Cytoprotective Properties in Different Cell Lines. Front. Pharmacol. 2021, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; Choya-Foces, C.; Hugo, M.; López, V. The Metabolite Urolithin-A Ameliorates Oxidative Stress in Neuro-2a Cells, Becoming a Potential Neuroprotective Agent. Antioxidants 2020, 9, 16. [Google Scholar] [CrossRef]
- OECD. OECD test No. 432: In vitro 3T3 NRU phototoxicity test. In OECD Guidelines for the Testing of Chemicals, Section 4; Organization for Economic Cooperation and Development (OECD): Paris, France, 2019. [Google Scholar] [CrossRef]
- Ferreira, I.; Silva, A.; Martins, J.D.; Neves, B.M.; Cruz, M.T. Nature and kinetics of redox imbalance triggered by respiratory and skin chemical sensitizers on the human monocytic cell line THP-1. Redox Biol. 2018, 16, 75–86. [Google Scholar] [CrossRef]
- Ding, Z.T.; Fang, Y.S.; Tai, Z.G.; Yang, M.H.; Xu, Y.Q.; Li, F.; Cao, Q.E. Phenolic content and radical scavenging capacity of 31 species of ferns. Fitoterapia 2008, 79, 581–583. [Google Scholar] [CrossRef]
- Xia, X.; Cao, J.G.; Zheng, Y.X.; Wang, Q.X.; Xiao, J.B. Flavonoid concentrations and bioactivity of flavonoid extracts from 19 species of ferns from China. Ind. Crops Prod. 2014, 58, 91–98. [Google Scholar] [CrossRef]
- García, F.; Pivel, J.P.; Guerrero, A.; Brieva, A.; Martínez-Alcázar, M.; Caamaño-Somoza, M.; González, S. Phenolic components and antioxidant activity of Fernblock®, an aqueous extract of the aerial parts of the fern Polypodium leucotomos. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 157–160. [Google Scholar] [CrossRef]
- Palomino, O.M. Current knowledge in Polypodium leucotomos effect on skin protection. Arch. Dermatol. Res. 2014, 307, 199–209. [Google Scholar] [CrossRef]
- Gombau, L.; Garcia, F.; Lahoz, A.; Fabre, M.; Roda-Navarro, P.; Majano, P.; Alonso-Lebrero, J.L.; Pivel, J.P.; Castell, J.V.; Gomez-Lechon, M.J.; et al. Polypodium leucotomos extract: Antioxidant activity and disposition. Toxicol. Vitr. 2006, 20, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Tomsík, P. Ferns and Lycopods-A Potential Treasury of Anticancer Agents but Also a Carcinogenic Hazard. Phytother. Res. 2014, 28, 798–810. [Google Scholar] [CrossRef]
- Iwashina, T.; Matsumoto, S. Flavonoid Properties of Six Asplenium Species in Vanuatu and New Caledonia, and Distribution of Flavonoid and Related Compounds in Asplenium. Bull. Natl. Mus. Nat. Sci. Ser. B. 2011, 37, 133–145. [Google Scholar]
- Bae, J.; Nayoung, K.; Yunyoung, S.; Soo-Yeon, K.; You-Jeong, K. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 1–10. [Google Scholar] [CrossRef]
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef]
- Morais-Braga, M.F.B.; Souza, T.M.; Santos, K.K.A.; Guedes, G.M.M.; Andrade, J.C.; Vega, C.; Rolón, M.; Costa, J.G.M.; Saraiva, A.A.F.; Coutinho, H.D.M. Phenol composition, cytotoxic and anti-kinetoplastidae activities of Lygodium venustum SW. (Lygodiaceae). Exp. Parasitol. 2013, 134, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Iwashina, T.; Matsumoto, S.; Ozawa, K.; Akuzawa, K. Flavone Glycosides from Asplenium Normale. Phytochemistry 1990, 29, 3543–3546. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Sun, C.L.; Wu, Z.S.; Wang, Z.Y.; Zhang, H.C. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid. Based Complement. Altern. Med. 2015, 2015, 9. [Google Scholar] [CrossRef]
- Nogueira, D.R.; Mitjans, M.; Infante, M.R.; Vinardell, M.P. Comparative sensitivity of tumor and non-tumor cell lines as a reliable approach for in vitro cytotoxicity screening of lysine-based surfactants with potential pharmaceutical applications. Int. J. Pharm. 2011, 420, 51–58. [Google Scholar] [CrossRef]
- Clothier, R.; Gómez-Lechón, M.J.; Kinsner-Ovaskainen, A.; Kopp-Schneider, A.; O’Connor, J.E.; Prieto, P.; Stanzel, S. Comparative analysis of eight cytotoxicity assays evaluated within the ACuteTox Project. Toxicol. Vitr. 2013, 27, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Bacanli, M.; Anlar, H.G.; Başaran, A.A.; Başaran, N. Assessment of Cytotoxicity Profiles of Different Phytochemicals: Comparison of Neutral Red and MTT Assays in Different Cells in Different Time Periods. Turk. J. Pharm. Sci. 2017, 14, 95–107. [Google Scholar] [CrossRef]
- Van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Karakas, D.; Ari, F.; Ulukaya, E. The MTT viability assay yields strikingly false-positive viabilities although the cells are killed by some plant extracts. Turk. J. Biol. 2017, 41, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Stokes, W.S.; Casati, S.; Strickland, J.; Paris, M. Neutral red uptake cytotoxicity tests for estimating starting doses for acute oral toxicity tests. Curr. Protoc. Toxicol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Ugartondo, V.; Mitjans, M.; Torres, J.L.; Vinardell, M.P. Biobased Epicatechin Conjugates Protect Erythrocytes and Nontumoral Cell Lines from H2O2-Induced Oxidative Stress. J. Agric. Food Chem. 2009, 57, 4459–4465. [Google Scholar] [CrossRef]
- Dutta, D.; Markhoff, J.; Suter, N.; Rezwan, K.; Brüggemann, D. Effect of Collagen Nanofibers and Silanization on the Interaction of HaCaT Keratinocytes and 3T3 Fibroblasts with Alumina Nanopores. Acs Appl. Bio Mater. 2021, 4, 1852–1862. [Google Scholar] [CrossRef]
- Clericuzio, M.; Burlando, B.; Gandini, G.; Tinello, S.; Ranzato, E.; Martinotti, S.; Cornara, L. Keratinocyte wound healing activity of galactoglycerolipids from the fern Ophioglossum vulgatum L. J. Nat. Med. 2014, 68, 31–37. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Tourino, S.; Torres, J.L.; Vinardell, M.P. Comparative Antioxidant and Cytotoxic Effect of Procyanidin Fractions from Grape and Pine. Chem. Res. Toxicol. 2007, 20, 1543–1548. [Google Scholar] [CrossRef]
- Babich, H.; Zuckerbraun, H.L.; Weinerman, S.M. In vitro cytotoxicity of (-)-catechin gallate, a minor polyphenol in green tea. Toxicol. Lett. 2007, 171, 171–180. [Google Scholar] [CrossRef]
- Petkov, V.; Batsalova, T.; Stoyanov, P.; Mladenova, T.; Kolchakova, D.; Argirova, M.; Raycheva, T.; Dzhambazov, B. Selective Anticancer Properties, Proapoptotic and Antibacterial Potential of Three Asplenium Species. Plants 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.; Schmittlandgraf, R.; Siegemund, B. Development of an in Vitro Test System with Human Skin Cells for Evaluation of Phototoxicity. Toxicol. Vitr. 1991, 5, 457–461. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Kashyap, M.P.; Kumar, V.; Tripathi, V.K.; Khanna, V.K.; Yadav, S.; Pant, A.B. Differential protection of pre-, co- and post-treatment of curcumin against hydrogen peroxide in PC12 cells. Hum. Exp. Toxicol. 2011, 30, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Koch, E. Complex Interactions between Phytochemicals. The Multi-Target Therapeutic Concept of Phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, F.; Xiao, M. Comparison of Phototoxicity Sensitivity by the Neutral Red Uptake Method for BALB/c 3T3, HaCaT, and HDFa Cells In Vitro. Innov. Digit. Health Diagn. Biomark. 2021, 2, 7. [Google Scholar] [CrossRef]
- Alonso-Lebrero, J.L.; Dominguez-Jimenez, C.; Tejedor, R.; Brieva, A.; Pivel, J.P. Photoprotective properties of a hydrophilic extract of the fern Polypodium leucotomos on human skin cells. J. Photochem. Photobiol. B-Biol. 2003, 70, 31–37. [Google Scholar] [CrossRef]
- Dasiman, R.; Nor, N.M.; Eshak, Z.; Mutalip, S.S.M.; Suwandi, N.R.; Bidin, H. A Review of Procyanidin: Updates on Current Bioactivities and Potential Health Benefits. Biointerface Res. Appl. Chem. 2022, 12, 5918–5940. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Erratum: Structure-antioxidant activity relationships of flavonoids and phenolic acids (Free Radical Biology and Medicine (1996) 20 (933–956)). Free. Radic. Biol. Med. 1996, 21, 417. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Wen, L.R.; He, J.R.; Wu, D.; Jiang, Y.M.; Prasad, K.N.; Zhao, M.M.; Lin, S.; Jiang, G.X.; Luo, W.; Yang, B. Identification of sesquilignans in litchi (Litchi chinensis Sonn.) leaf and their anticancer activities. J. Funct. Foods 2014, 8, 26–34. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.E.; Caricato, R.; Lionetto, M.G. Concentration Dependence of the Antioxidant and Prooxidant Activity of Trolox in HeLa Cells: Involvement in the Induction of Apoptotic Volume Decrease. Antioxidants 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Yen, W.-J.; Chyau, C.-C.; Lee, C.-P.; Chu, H.-L.; Chang, L.-W.; Duh, P.-D. Cytoprotective effect of white tea against H2O2-induced oxidative stress in vitro. Food Chem. 2013, 141, 4107–4114. [Google Scholar] [CrossRef]
- Zivkovic, S.; Milutinovic, M.; Maksimovi, V.; Ciric, A.; Ivanov, M.; Bozunovic, J.; Banjanac, T.; Misic, D. Antioxidant and antimicrobial activity of two Asplenium species. South Afr. J. Bot. 2020, 132, 180–187. [Google Scholar] [CrossRef]






| N° | Phytochemicals | Methanolic Extract Asplenium adiantum-nigrum L. Fronds (AAM) |
|---|---|---|
| Phenolic acids | ||
| 1 | Gallic acid | 30.56 |
| 2 | Neochlorogenic acid | n.d. |
| 3 | Chlorogenic acid | 681.10 |
| 4 | p-Hydroxybenzoic acid | 109.38 |
| 5 | 3-Hydroxy benzoic acid | n.d. |
| 6 | Caffeic acid | 16.68 |
| 7 | Vanillic acid | 235.19 |
| 8 | Syringic acid | n.d. |
| 9 | p-Coumaric acid | 61.10 |
| 10 | Ferulic acid | 1.77 |
| 11 | 3,5-Dicaffeoylquinic acid | 3.62 |
| 12 | Ellagic acid | n.d. |
| Flavonoids | ||
| (A) Anthocyanins | ||
| 13 | Delphinidin-3,5-diglucoside | 2.06 |
| 14 | Delphinidin-3-galactoside | 0.52 |
| 15 | Cyanidin-3-glucoside | 64.07 |
| 16 | Petunidin-3-glucoside | n.d. |
| 17 | Pelargonidin-3-rutinoside | n.d. |
| 18 | Pelargonidin-3-glucoside | n.d. |
| 19 | Malvidin-3-galactoside | n.d. |
| (B) Flavonols | ||
| 20 | Rutin | 5.54 |
| 21 | Isoquercitrin | 3.17 |
| 22 | Quercitrin | 0.99 |
| 23 | Myricetin | 0.71 |
| 24 | Kaempferol-3-glucoside | 36.85 |
| 25 | Quercetin | 0.74 |
| 26 | Isorhamnetin | 0.18 |
| 27 | Hyperoside | 5.59 |
| 28 | Kaempferol | 1.88 |
| (C) Flavan-3-ols (Flavanols) | ||
| 29 | Catechin | n.d. |
| 30 | Epicatechin | 7.69 |
| 31 | Procyanidin B2 | 110.16 |
| 32 | Procyanidin A2 | 3.63 |
| (D) Dihydrochalcones | ||
| 33 | Phloridzin | n.d. |
| 34 | Phloretin | n.d. |
| (E) Flavanones | ||
| 35 | Hesperidin | 14.81 |
| 36 | Naringin | n.d. |
| Stilbenes | ||
| 37 | Resveratrol | n.d. |
| Non-phenolic acids | ||
| 38 | Trans-cinnamic acid | 7.69 |
| Total phenol content | 1405.68 |
| Concentration of AAM (mg/mL) | 0.01 | 0.1 | 1 |
|---|---|---|---|
| Cytoprotection activity (%) a in 3T3 | 8.7% | 27.7% | 55.6% |
| Cytoprotection activity (%) a in HaCaT | 0.0% | 0.0% | 4.4% |
| Concentration of AAM (mg/mL) | 0.01 | 0.1 | 1 |
|---|---|---|---|
| Cellular repair activity (%) a | 18.8% | 41.6% | 77.5% |
| Cell Line | 3T3 | HaCaT | ||||
|---|---|---|---|---|---|---|
| Concentration of AAM (mg/mL) | 0.01 | 0.1 | 1 | 0.01 | 0.1 | 1 |
| 1 mM H2O2 | 19.6 | 22.7 | 34.0 | 37.5 | 79.7 | 93.1 |
| 2 mM H2O2 | 3.8 | 40.8 | 30.6 | 22.8 | 46.4 | 86.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farràs, A.; López, V.; Maggi, F.; Caprioli, G.; Vinardell, M.P.; Mitjans, M. Chemical Composition and Cytoprotective Activities of Methanolic Extract of Asplenium adiantum-nigrum L. (Aspleniaceae). Horticulturae 2022, 8, 815. https://doi.org/10.3390/horticulturae8090815
Farràs A, López V, Maggi F, Caprioli G, Vinardell MP, Mitjans M. Chemical Composition and Cytoprotective Activities of Methanolic Extract of Asplenium adiantum-nigrum L. (Aspleniaceae). Horticulturae. 2022; 8(9):815. https://doi.org/10.3390/horticulturae8090815
Chicago/Turabian StyleFarràs, Adrià, Víctor López, Filippo Maggi, Giovanni Caprioli, María Pilar Vinardell, and Montserrat Mitjans. 2022. "Chemical Composition and Cytoprotective Activities of Methanolic Extract of Asplenium adiantum-nigrum L. (Aspleniaceae)" Horticulturae 8, no. 9: 815. https://doi.org/10.3390/horticulturae8090815