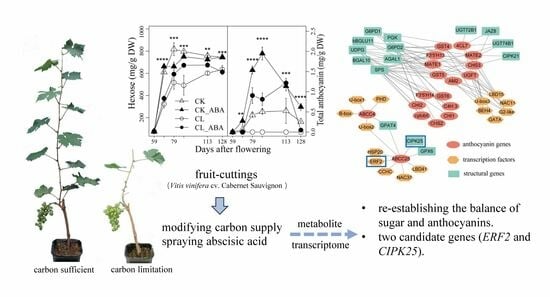
Transcriptome Remodeling in Response to Leaf Removal and Exogenous Abscisic Acid in Berries of Grapevine (Vitis vinifera L.) Fruit Cuttings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Treatments, and Sampling
2.2. Extraction and Determination of Soluble Sugars
2.3. Extraction and Determination of Anthocyanins
2.4. Transcriptome Sequencing and RNA Sequencing Data Analysis
2.5. Network Analysis and Visualization
2.6. Statistical Analysis
3. Results
3.1. The Effect of Carbon Limitation and Spraying ABA Treatment on Grape Berry Metabolites
3.2. mRNA Quantities of Genes Related to Anthocyanin Biosynthesis and Hormone Signaling Pathways
3.3. Transcriptomic Response of Berries to Exogenous ABA Treatment under Different Carbon Supplies
3.4. Transcriptomic Response of Berries to Carbon Limitation under ABA or Non-ABA Treatment
3.5. The Correlation Network of Genes Related to Anothocynain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CK | carbon sufficient |
| CL | carbon limitation |
| ABA | abscisic acid |
| CK_ABA | carbon sufficient and exogenous ABA treatment |
| CL_ABA | carbon limitation and exogenous ABA treatment |
References
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Duchene, E.; Schneider, C. Grapevine and climatic changes: A glance at the situation in Alsace. Agron. Sustain. Dev. 2005, 25, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Yamane, T.; Jeong, S.T.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am. J. Enol. Vitic. 2006, 57, 54–59. [Google Scholar]
- Lecourieux, F.; Kappel, C.; Pieri, P.; Charon, J.; Pillet, J.; Hilbert, G.; Renaud, C.; Gomes, E.; Delrot, S.; Lecourieux, D. Dissecting the biochemical and transcriptomic effects of a locally applied heat treatment on developing Cabernet Sauvignon grape berries. Front. Plant Sci. 2017, 8, 00053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, R.; Rana, V. Manipulation of source-sink relationship in pertinence to better fruit quality and yield in fruit crops: A review. Agric. Rev. 2019, 40, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, M.P.; Lillo-Carmona, V.; Melet, L.; Benedetto, G.; Andrade, D.; Maucourt, M.; Deborde, C.; Fuentealba, C.; Moing, A.; Valenzuela, M.L.; et al. Metabolite fruit profile is altered in response to source-sink imbalance and can be used as an early predictor of fruit quality in nectarine. Front. Plant Sci. 2021, 11, 4133–4146. [Google Scholar] [CrossRef] [PubMed]
- Aslani, L.; Gholami, M.; Mobli, M.; Ehsanzadeh, P.; Bertin, N. Decreased sink/source ratio enhances hexose transport in the fruits of greenhouse tomatoes: Integration of gene expression and biochemical analyses. Physiol. Plant. 2020, 170, 120–131. [Google Scholar] [CrossRef]
- Bairam, E.; leMorvan, C.; Delaire, M.; Buck-Sorlin, G. Fruit and leaf response to different source-sink ratios in apple, at the scale of the fruit-bearing branch. Plant Sci. 2019, 10, 1039–1052. [Google Scholar] [CrossRef]
- Bogicevic, M.; Maras, V.; Mugosa, M.; Kodzulovic, V.; Raicevic, J.; Sucur, S.; Failla, O. The effects of early leaf removal and cluster thinning treatments on berry growth and grape composition in cultivars Vranac and Cabernet Sauvignon. Chem. Biol. Technol. Agric. 2015, 2, 13–20. [Google Scholar] [CrossRef] [Green Version]
- VanderWeide, J.; Gottschalk, C.; Schultze, S.R.; Nasrollahiazar, E.; Poni, S.; Sabbatini, P. Impacts of pre-bloom leaf removal on wine grape production and quality parameters: A systematic review and meta-analysis. Front. Plant Sci. 2021, 11, 621585. [Google Scholar] [CrossRef]
- Bobeica, N.; Poni, S.; Hilbert, G.; Renaud, C.; Gomes, E.; Delrot, S.; Dai, Z.W. Differential responses of sugar, organic acids and anthocyanins to source-sink modulation in Cabernet Sauvignon and Sangiovese grapevines. Front. Plant Sci. 2015, 6, 00382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulker, T.; Kamiloglu, M.U. Influences of girdling and potassium treatments on fruit quality and some physiological characters of ‘Fremont’ mandarin variety. Folia Hortic. 2021, 33, 195–202. [Google Scholar] [CrossRef]
- Nardozza, S.; Boldingh, H.L.; Kashuba, M.P.; Feil, R.; Jones, D.; Thrimawithana, A.H.; Ireland, H.S.; Philippe, M.; Wohlers, M.W.; McGhie, T.K.; et al. Carbon starvation reduces carbohydrate and anthocyanin accumulation in red-fleshed fruit via trehalose 6-phosphate and MYB27. Plant Cell Environ. 2020, 43, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Hikosaka, S.; Kobayshi, M.; Nishizawa, T.; Saito, K.; Goto, E.; Kusano, M. A systems analysis with “simplified source-sink model” reveals metabolic reprogramming in a pair of source-to-sink organs during early fruit development in tomato by LED light treatments. Front. Plant Sci. 2018, 9, 01439. [Google Scholar] [CrossRef]
- Pastore, C.; Allegro, G.; Valentini, G.; Muzzi, E.; Filippetti, I. Anthocyanin and flavonol composition response to veraison leaf removal on Cabernet Sauvignon, Nero d’Avola, Raboso Piave and Sangiovese Vitis vinifera L. cultivars. Sci. Hortic. 2017, 218, 147–155. [Google Scholar] [CrossRef]
- Diago, M.P.; Ayestaran, B.; Guadalupe, Z.; Poni, S.; Tardaguila, J. Impact of prebloom and fruit set basal leaf removal on the flavonol and anthocyanin composition of tempranillo grapes. Am. J. Enol. Vitic. 2012, 63, 367–376. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gray, D.J.; Lu, J.A.; Gu, L.W. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 2011, 126, 982–988. [Google Scholar] [CrossRef]
- Feng, H.; Skinkis, P.A.; Qian, M.C. Pinot noir wine volatile and anthocyanin composition under different levels of vine fruit zone leaf removal. Food Chem. 2017, 214, 736–744. [Google Scholar] [CrossRef]
- Pastore, C.; Zenoni, S.; Fasoli, M.; Pezzotti, M.; Tornielli, G.B.; Filippetti, I. Selective defoliation affects plant growth, fruit transcriptional ripening program and flavonoid metabolism in grapevine. BMC Plant Biol. 2013, 13, 30–45. [Google Scholar] [CrossRef] [Green Version]
- Intrieri, C.; Filippetti, I.; Allegro, G.; Centinari, M.; Poni, S. Early defoliation (hand vs mechanical) for improved crop control and grape composition in Sangiovese (Vitis vinifera L.). Aust. J. Grape Wine Res. 2008, 14, 25–32. [Google Scholar] [CrossRef]
- Ferrandino, A.; Guidoni, S.; Mannini, F. Grape quality parameters and polyphenolic content of different ‘Barbera’ and ‘Nebbiolo’ (Vitis vinifera L.) clones as influenced by environmental conditions—Preliminary results. Acta Hortic. 2007, 754, 437–442. [Google Scholar] [CrossRef]
- Wang, L.N.; Brouard, E.; Hilbert, G.; Renaud, C.; Petit, J.P.; Edwards, E.; Betts, A.; Delrot, S.; Guillaumie, S.; Gomes, E.; et al. Differential response of the accumulation of primary and secondary metabolites to leaf-to-fruit ratio and exogenous abscisic acid. Aust. J. Grape Wine Res. 2021, 27, 527–539. [Google Scholar] [CrossRef]
- Wang, L.N.; Brouard, E.; Prodhomme, D.; Hilbert, G.; Renaud, C.; Petit, J.-P.; Edwards, E.; Betts, A.; Delrot, S.; Ollat, N.; et al. Regulation of anthocyanin and sugar accumulation in grape berry through carbon limitation and exogenous ABA application. Food Res. Int. 2022, 160, 111478. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.H.; Niu, N.; Li, J.H.; Li, S.H. Leaf:fruit ratio affects the proteomic profile of grape berry skins. J. Am. Soc. Hortic. Sci. 2013, 138, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Guidoni, S.; Ferrandino, A.; Novello, V. Effects of seasonal and agronomical practices on skin anthocyanin profile of Nebbiolo grapes. Am. J. Enol. Vitic. 2008, 59, 22–29. [Google Scholar]
- Jia, H.F.; Xie, Z.Q.; Wang, C.; Shangguan, L.F.; Qian, N.; Cui, M.J.; Liu, Z.J.; Zheng, T.; Wang, M.Q.; Fang, J.G. Abscisic acid, sucrose, and auxin coordinately regulate berry ripening process of the Fujiminori grape. Funct. Integr. Genom. 2017, 17, 441–457. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Matthews, M.A.; Shaghasi, T.H.; McElrone, A.J.; Castellarin, S.D. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 2010, 232, 219–234. [Google Scholar] [CrossRef] [Green Version]
- Olivares, D.; Contreras, C.; Munoz, V.; Rivera, S.; Gonzalez-Aguero, M.; Retamales, J.; Defilippi, B.G. Relationship among color development, anthocyanin and pigment-related gene expression in ‘Crimson Seedless’ grapes treated with abscisic acid and sucrose. Plant Physiol. Biochem. 2017, 115, 286–297. [Google Scholar] [CrossRef]
- Koyama, R.; Roberto, S.R.; de Souza, R.T.; Borges, W.F.S.; Anderson, M.; Waterhouse, A.L.; Cantu, D.; Fidelibus, M.W.; Blanco-Ulate, B. Exogenous abscisic acid promotes anthocyanin biosynthesis and increased expression of flavonoid synthesis genes in Vitis vinifera x Vitis labrusca table grapes in a subtropical region. Front. Plant Sci. 2018, 9, 00323. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.L.; Liu, Q.Z.; Xi, B.; Dai, H.J. Study on the regulation of anthocyanin biosynthesis by exogenous abscisic acid in grapevine. Sci. Hortic. 2019, 250, 294–301. [Google Scholar] [CrossRef]
- Mullins, M.G.; Rajasekaran, K. Fruiting cuttings: Revised method for producing test plants of grapevine cultivars. Am. J. Enol. Vitic. 1981, 32, 35–40. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 32. [Google Scholar]
- Kuang, Y.F.; Ren, C.; Wang, Y.; Kirabi, G.E.; Wang, Y.J.; Wang, L.J.; Fan, P.G.; Liang, Z.C. Characterization of the berry quality traits and metabolites of ‘Beimei’ interspecific hybrid wine grapes during berry development and winemaking. Horticulturae 2022, 8, 516. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, L.M.; Ren, C.; Ren, F.R.; Wang, Y.; Fan, P.G.; Li, S.H.; Liang, Z.C. VvSWEET10 mediates sugar accumulation in grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.C.; Wu, B.H.; Fan, P.G.; Yang, C.X.; Duan, W.; Zheng, X.B.; Liu, C.Y.; Li, S.H. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chem. 2008, 111, 837–844. [Google Scholar] [CrossRef]
- Dai, Z.W.; Meddar, M.; Renaud, C.; Merlin, I.; Hilbert, G.; Delrot, S.; Gomes, E. Long-term in vitro culture of grape berries and its application to assess the effects of sugar supply on anthocyanin accumulation. J. Exp. Bot. 2014, 65, 4665–4677. [Google Scholar] [CrossRef]
- Hilbert, G.; Soyer, J.P.; Molot, C.; Giraudon, J.; Milin, S.; Gaudillere, J.P. Effects of nitrogen supply on must quality and anthocyanin accumulation in berries of cv. Merlot. Vitis 2003, 42, 69–76. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Z.; Hu, E.Q.; Xu, S.B.; Chen, M.J.; Guo, P.F.; Dai, Z.H.; Feng, T.Z.; Zhou, L.; Tang, W.L.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ranmage, D.; Amin, N.; Schwikowski, B.; Trey, I. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bigard, A.; Romieu, C.; Ojeda, H.; Torregrosa, L. The sugarless grape trait characterised by single berry phenotyping. OENO One 2022, 56, 89–102. [Google Scholar] [CrossRef]
- Martinez de Toda, F.; Balda, P. Delaying berry ripening through manipulating leaf area to fruit ratio. Vitis 2013, 52, 171–176. [Google Scholar]
- Arnold, T.; Appel, H.; Patel, V.; Stocum, E.; Kavalier, A.; Schultz, J. Carbohydrate translocation determines the phenolic content of Populus foliage: A test of the sink-source model of plant defense. New Phytol. 2004, 164, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, E.; Matus, J.T.; Finezzo, L.; Zenoni, S.; Loyola, R.; Guzzo, F.; Schlechter, R.; Ageorges, A.; Arce-Johnson, P.; Tornielli, G.B. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine. Plant Physiol. 2015, 167, 1448–1470. [Google Scholar] [CrossRef]
- He, L.; Ren, Z.Y.; Wang, Y.; Fu, Y.Q.; Li, Y.; Meng, N.; Pan, Q.H. Variation of growth-to-ripening time interval induced by abscisic acid and synthetic auxin affecting transcriptome and flavor compounds in Cabernet Sauvignon grape berry. Plants 2020, 9, 630. [Google Scholar] [CrossRef]
- Koyama, K.; Sadamatsu, K.; Goto-Yamamoto, N. Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Funct. Integr. Genom. 2010, 10, 367–381. [Google Scholar] [CrossRef] [Green Version]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, M.; Dal Santo, S.; Zenoni, S.; Tornielli, G.B.; Farina, L.; Zamboni, A.; Porceddu, A.; Venturini, L.; Bicego, M.; Murino, V.; et al. The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 2012, 24, 3489–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.Y.; Yang, T.; Li, Y.; Zhang, J.; Wu, T.; Song, T.T.; Yao, Y.C.; Tian, J. The long noncoding RNA MdLNC499 bridges MdWRKY1 and MdERF109 function to regulate early-stage light-induced anthocyanin accumulation in apple fruit. Plant Cell 2021, 33, 3309–3330. [Google Scholar] [CrossRef]
- An, J.P.; Zhang, X.W.; Bi, S.Q.; You, C.X.; Wang, X.F.; Hao, Y.J. The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple. Plant J. 2020, 101, 573–589. [Google Scholar] [CrossRef]
- Ni, J.; Bai, S.; Zhao, Y.; Qian, M.; Tao, R.; Yin, L.; Gao, L.; Teng, Y. Ethylene response factors Pp4ERF24 and Pp12ERF96 regulate blue light-induced anthocyanin biosynthesis in ‘Red Zaosu’ pear fruits by interacting with MYB114. Plant Mol. Biol. 2019, 99, 67–78. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, J.; Dong, C.; Cheng, Z.M. The CBL and CIPK Gene family in grapevine (Vitis vinifera): Genome-wide analysis and expression profiles in response to various abiotic stresses. Front. Plant Sci. 2017, 8, 00978. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, Y.; Xiong, L. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol 2007, 144, 1416–1428. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Liu, B.W.; Sun, X.Z.; Sun, X.; Zheng, C.S. New functions of CIPK gene family are continue to emerging. Mol. Biol. Rep. 2022, 49, 6647–6658. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, T.C.; Li, L.L.; Zhuo, X.K.; Jiang, L.B.; Jia, W.; Cheng, T.R.; Zhang, X.Q. Identification and comparative analysis of the CIPK gene family and characterization of the cold stress response in the woody plant Prunus mume. PeerJ 2019, 7, 6847–6869. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Liu, Y.J.; You, C.X.; Hao, Y.J. An apple CIPK protein kinase targets a novel residue of AREB transcription factor for ABA-dependent phosphorylation. Plant Cell Environ. 2017, 40, 2207–2219. [Google Scholar] [CrossRef]
- Yan, J.; Niu, F.; Liu, W.Z.; Zhang, H.; Wang, B.; Yang, B.; Jiang, Y.Q. Arabidopsis CIPK14 positively regulates glucose response. Biochem. Biophys. Res. Commun. 2014, 450, 1679–1685. [Google Scholar] [CrossRef]
- Hu, D.G.; Sun, C.H.; Zhang, Q.Y.; An, J.P.; You, C.X.; Hao, Y.J. Glucose sensor MdHXK1 phosphorylates and stabilizes MdbHLH3 to promote anthocyanin biosynthesis in apple. PLos Genet. 2016, 12, 1006273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.F.; Jiu, S.T.; Zhang, C.; Wang, C.; Tariq, P.; Liu, Z.J.; Wang, B.J.; Cui, L.W.; Fang, J.G. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol. J. 2016, 14, 2045–2065. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.K.; Zhao, Y.W.; Sun, C.H.; Hu, D.G. Deciphering the impact of glucose signaling on fruit quality. Fruit Res. 2022, 2, 3–8. [Google Scholar] [CrossRef]
- Duran-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Q.; Liu, L.; Zhao, Y.; Kong, J.; Wang, Y.; Xu, X.; Hilbert, G.; Gomès, E.; Dai, Z. Transcriptome Remodeling in Response to Leaf Removal and Exogenous Abscisic Acid in Berries of Grapevine (Vitis vinifera L.) Fruit Cuttings. Horticulturae 2022, 8, 905. https://doi.org/10.3390/horticulturae8100905
Tong Q, Liu L, Zhao Y, Kong J, Wang Y, Xu X, Hilbert G, Gomès E, Dai Z. Transcriptome Remodeling in Response to Leaf Removal and Exogenous Abscisic Acid in Berries of Grapevine (Vitis vinifera L.) Fruit Cuttings. Horticulturae. 2022; 8(10):905. https://doi.org/10.3390/horticulturae8100905
Chicago/Turabian StyleTong, Qian, Li Liu, Yan Zhao, Junhua Kong, Yongjian Wang, Xiaobo Xu, Ghislaine Hilbert, Eric Gomès, and Zhanwu Dai. 2022. "Transcriptome Remodeling in Response to Leaf Removal and Exogenous Abscisic Acid in Berries of Grapevine (Vitis vinifera L.) Fruit Cuttings" Horticulturae 8, no. 10: 905. https://doi.org/10.3390/horticulturae8100905