Assessing the Effect of Plant Growth Stimulants and Retardants on Cyclamen “Halios F1 Salmon Rose” Cultivar
Abstract
:1. Introduction
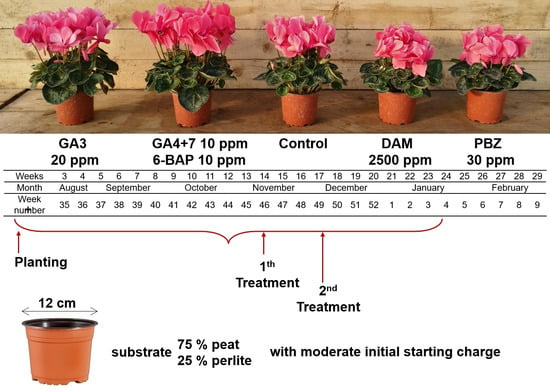
2. Materials and Methods
Data Analyses
3. Results
3.1. Rosette Diameter under the Effect of PGRs and Plant Growth Regulators
3.2. Rosette Height Influenced by PGRs and Plant Growth Regulators
3.3. Peduncle Height under the Effect of PGRs and Plant Growth Regulators
3.4. Peduncle Diameter Influenced by PGR’s and Plant Growth Regulator
3.5. Plant Height under the Effect of PGRs and Plant Growth Regulators
3.6. Number of Flower Buds Influenced by PGRs and Plant Growth Regulators
3.7. Number of Flowers under the Effect of PGRs and Plant Growth Regulators
3.8. Inflorescence Diameter Influenced by PGRs and Plant Growth Regulators
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Turiné Farkas, Z.; Palkovics, A.; Jóljárt, F. Analysis of Growing Cyclamen persicum. Gradus 2020, 7, 43–49. [Google Scholar] [CrossRef]
- Van Huylenbroeck, J. Status of Floriculture in Europe. In Protocols for In Vitro Propagation of Ornamental Plants; Jain, S.M., Ochatt, S.J., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 589, pp. 365–376. ISBN 978-1-60327-390-9. [Google Scholar]
- Darras, A.I. Implementation of Sustainable Practices to Ornamental Plant Cultivation Worldwide: A Critical Review. Agronomy 2020, 10, 1570. [Google Scholar] [CrossRef]
- Karlsson, M.; Werner, J. Temperature Affects Leaf Unfolding Rate and Flowering of Cyclamen. HortScience 2001, 36, 292–294. [Google Scholar] [CrossRef]
- Winkelmann, T. Clonal Propagation of Cyclamen persicum via Momatic Embryogenesis. In Protocols for In Vitro Propagation of Ornamental Plants; Jain, S.M., Ochatt, S.J., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 589, pp. 281–290. ISBN 978-1-60327-390-9. [Google Scholar]
- Seyring, M.; Ewald, A.; Mueller, A.; Haensch, K.-T. Screening for Propagation Suitability in Vitro of Different Cyclamen Species. Electron. J. Biotechnol. 2009, 12, 1–11. [Google Scholar] [CrossRef]
- Foubert, K.; Theunis, M.; Apers, S.; Vlietinck, A.; Pieters, L. Chemistry, Distribution and Biological Activities of 13,28-Epoxy-Oleanane Saponins from the Plant Families Myrsinaceae and Primulaceae. Curr. Org. Chem. 2008, 12, 629–642. [Google Scholar] [CrossRef]
- Quave, C.L.; Plano, L.R.W.; Pantuso, T.; Bennett, B.C. Effects of Extracts from Italian Medicinal Plants on Planktonic Growth, Biofilm Formation and Adherence of Methicillin-Resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef]
- Rode, C.; Gallien, S.; Heintz, D.; Van Dorsselaer, A.; Braun, H.-P.; Winkelmann, T. Enolases: Storage Compounds in Seeds? Evidence from a Proteomic Comparison of Zygotic and Somatic Embryos of Cyclamen persicum Mill. Plant Mol. Biol. 2011, 75, 305–319. [Google Scholar] [CrossRef]
- Takamura, T. Cyclamen. In Flower Breeding and Genetics; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 459–478. ISBN 978-1-4020-4427-4. [Google Scholar]
- Compton, J.A.; Clennett, J.C.B.; Culham, A. Nomenclature in the Dock. Overclassification Leads to Instability: A Case Study in the Horticulturally Important Genus Cyclamen (Myrsinaceae). Bot. J. Linn. Soc. 2004, 146, 339–349. [Google Scholar] [CrossRef]
- Yesson, C.; Culham, A. A Phyloclimatic Study of Cyclamen. BMC Ecol. Evol. 2006, 6, 72. [Google Scholar] [CrossRef]
- Jalali, N.; Naderi, R.; Shahi-Gharahlar, A.; da Silva, J.A.T. Tissue Culture of Cyclamen spp. Sci. Hortic. 2012, 137, 11–19. [Google Scholar] [CrossRef]
- Schwartz-Tzachor, R.; Eisikowitch, D.; Dafni, A. Flower Characteristics and Breeding System of Two Phenological Ecotypes of Cyclamen persicum Mill. (Myrsinaceae) in Israel. Plant Syst. Evol. 2008, 274, 127–134. [Google Scholar] [CrossRef]
- Terakawa, T.; Yamamura, T.; Murayama, T. Improvement of Regeneration and Transformation Systems for Cyclamen persicum Using Somatic Embryo Culture. Plant Biotechnol. 2008, 25, 77–80. [Google Scholar] [CrossRef]
- Pavlíček, T.; Bureš, P.; Horová, L.; Raskina, O.; Nevo, E. Genome Size Microscale Divergence of Cyclamen persicum in Evolution Canyon, Israel. Open Life Sci. 2008, 3, 83–90. [Google Scholar] [CrossRef]
- Savona, M.; Ruffoni, B.; Giovannini, A.; Altamura, M.M. Cyclamen persicum Mill Cv. “Halios”: Somatic Embryogenesis and Phenotypic Analysis of Somatic Embryo-Derived Plants. Acta Hortic. 2007, 91–97. [Google Scholar] [CrossRef]
- Bala, M.; Berecici, D. The Behaviour of Some Old Cyclamen persicum Varieties in Growing Conditions of a Modern Greenhouse from the Didactic Base of the Faculty of Horticulture and Forestry in Timişoara. Bull. Univ. Agric. Sci. Vet. 2010, 67, 298–301. [Google Scholar]
- Lewis, K.P.; Faust, J.E.; Sparkman, J.D.; Grimes, L.W. The Effect of Daminozide and Chlormequat on the Growth and Flowering of Poinsettia and Pansy. HortScience 2004, 39, 1315–1318. [Google Scholar] [CrossRef]
- Batelja Lodeta, K.; Goreta Ban, S.; Perica, S.; Dumicic, G.; Bucan, L. Response of Poinsettia to Drench Application of Growth Regulators. J. Food Agric. Environ. 2010, 8, 297–301. [Google Scholar]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Bons, H.K.; Kaur, M. Role of Plant Growth Regulators in Improving Fruit Set, Quality and Yield of Fruit Crops: A Review. J. Hortic. Sci. Biotechnol. 2020, 95, 137–146. [Google Scholar] [CrossRef]
- Runkle, E.; Blancard, M.; Olrich, M. Fascination on Poinsettia. Available online: https://www.canr.msu.edu/resources/fascination-on-poinsettia (accessed on 17 February 2022).
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin Signaling Networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef]
- Markovich, O.; Steiner, E.; Kouřil, Š.; Tarkowski, P.; Aharoni, A.; Elbaum, R. Silicon Promotes Cytokinin Biosynthesis and Delays Senescence in Arabidopsis and Sorghum: Silicon and Cytokinin. Plant Cell Environ. 2017, 40, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Massolo, J.F.; Lemoine, M.L.; Chaves, A.R.; Concellón, A.; Vicente, A.R. Benzyl-Aminopurine (BAP) Treatments Delay Cell Wall Degradation and Softening, Improving Quality Maintenance of Refrigerated Summer Squash. Postharvest Biol. Technol. 2014, 93, 122–129. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, Y.C.; Kim, J.U.; Jo, I.H.; Kim, K.H.; Kim, D.H. Effects of gibberellic acid and alternating temperature on breaking seed dormancy of Panax ginseng CA Meyer. Korean J. Med. Crop. Sci. 2016, 24, 284–293. [Google Scholar] [CrossRef]
- Oh, W.; Kim, K.S. Light Intensity and Temperature Regulate Petiole Elongation by Controlling the Content of and Sensitivity to Gibberellin in Cyclamen persicum. Hortic. Environ. Biotechnol. 2014, 55, 175–182. [Google Scholar] [CrossRef]
- Alshakhaly, Z.M.; Qrunfleh, M.M. Effect of Plant Growth Regulators on Flower Development and Quality of Five Cyclamen persicum Hybrids. Acta Hortic. 2019, 215–222. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin Biosynthesis and Its Regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- An, K.B.; Lee, Y.S.; Son, K.C. Effect of Plant Growth Retardants on Producing High Quality of Cyclamen. Korean J. Hortic. Sci. 2004, 22, 65. [Google Scholar]
- Fletcher, R.A.; Gilley, A.; Sankhla, N.; Davis, T.D. Triazoles as Plant Growth Regulators and Stress Protectants. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Oxford, UK, 2010; pp. 55–138. ISBN 978-0-470-65077-6. [Google Scholar]
- Rostami, S.; Azhdarpoor, A. The Application of Plant Growth Regulators to Improve Phytoremediation of Contaminated Soils: A Review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef]
- García-Martinez, J.L.; Gil, J. Light Regulation of Gibberellin Biosynthesis and Mode of Action. J. Plant Growth Regul. 2001, 20, 354–368. [Google Scholar] [CrossRef]
- Tan, Z.G.; Qian, Y.L. Light Intensity Affects Gibberellic Acid Content in Kentucky Bluegrass. HortScience 2003, 38, 113–116. [Google Scholar] [CrossRef]
- Nishijima, T. Use of Plant Growth Regulators for Floriculture in Japan. Sci. Hortic. 2023, 309, 111630. [Google Scholar] [CrossRef]
- Niu, G.; Heins, R.; Carlson, W. Using Paclobutrazol to Control Height of Poinsettia ‘Freedom’. HortTechnology 2002, 12, 232–236. [Google Scholar] [CrossRef]
- Elisheba, B.P.; Sudghar, R. Growth Manipulation in Ornamental Sunflower (Helianthus annuus) Cv. Ring of Fire as a Bedding Plant. Crop Res. 2021, 56, 30–36. [Google Scholar] [CrossRef]
- Cornea-Cipcigan, M.; Pamfil, D.; Sisea, C.R.; Mărgăoan, R. Gibberellic Acid Can Improve Seed Germination and Ornamental Quality of Selected Cyclamen Species Grown under Short and Long Days. Agronomy 2020, 10, 516. [Google Scholar] [CrossRef]
- Kentelky, E.; Szekely-Varga, Z.; Bálint, J.; Balog, A. Enhance Growth and Flower Quality of Chrysanthemum Indicum l. with Application of Plant Growth Retardants. Horticulturae 2021, 7, 532. [Google Scholar] [CrossRef]
- Hg, M.; Dt, L.; Dt, B. Effects of Plant Growth Retardants and Pot Sizes on the Height of Potting Ornamental Plants: A Short Review. J. Hortic. 2018, 5. [Google Scholar] [CrossRef]
- Osterc, G.; Mikulic Petkovsek, M.; Stampar, F.; Ravnjak, B.; Bavcon, J. Impact of Specific Environmental Characteristics of the Site of Origin (Shady, Sunny) on Anthocyanin and Flavonol Contents of Replanted Plants at Common Cyclamen (Cyclamen purpurascens Mill.). Acta Physiol. Plant 2017, 39, 64. [Google Scholar] [CrossRef]
- Csorba, A.B.; Kentelky, E.; Szabó, M.-E.; Jakab, M.; Nyárádi, I.-I.; Bálint, J. Controlling Grey Mold (Botrytis cinerea) in Flowering Cyclamen Production. Europ. J. Hortic. Sci. 2023, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O.; Elad, Y.; Kirshner, B.; Volpin, H.; Zielsin, N. Control of Botrytis Cinerea in Cut Rose Flowers by Gibberellic Acid, Ethylene Inhibitors and Calcium. In Recent Advances in Botrytis Research; Verhoeff, K., Malathrakis, N.E., Williamson, B., Eds.; Pudoc Scientific Publishers: Wageningen, The Netherlands, 1992; pp. 257–261. [Google Scholar]
- Bosch, E.; Cuquel, F.L.; Tognon, G.B. Physalis Size Reduction for Potted Ornamental Plant Use. Ciência Agrotecnologia 2016, 40, 555–564. [Google Scholar] [CrossRef]
- Wanderley, C.D.S.; de Faria, R.T.; Ventura, M.U.; Vendrame, W. The Effect of Plant Growth Regulators on Height Control in Potted Arundina Graminifolia Orchids (Growth Regulators in Arundina Graminifolia). Acta Sci. Agron. 2014, 36, 489. [Google Scholar] [CrossRef]
- Bañón, S.; Ochoa, J.; Fernández, J.A.; González, A. Plant Growth Retardants for Introduction of Native Reichardia Tingitana. Acta Hortic. 2003, 271–277. [Google Scholar] [CrossRef]
- Ravnjak, B.; Bavcon, J.; Osterc, G. Physiological Response of Local Populations of Species Cyclamen purpurascens Mill. to Forest Gaps. Appl. Ecol. Env. Res. 2019, 17, 11489–11508. [Google Scholar] [CrossRef]
- Nowak, J. Wplyw roznych preparatow zawierajacych kwas giberelinowy na wzrost i kwitnienie cyklamena i gerbery. Zesz. Nauk. Inst. Sadow. I Kwiaciarstwa W Skiern. 2000, 7, 259–263. [Google Scholar]
- Sahu, J.K.; Tamrakar, S.K.; Lakpale, R.; Tirkey, T. Effect of Planting Geometry and Plant Growth Regulators on Growth and Flowering of Chrysanthemum. Progress. Hortic. 2021, 53, 105–108. [Google Scholar] [CrossRef]
- Malik, S.; Rather, Z.; Wani, M.; Din, A.; Nazki, I. Effect of Growth Regulators on Plant Growth and Flowering in Dahlia (Dahlia variabilis) Cv. Charmit. J. Exp. Agric. Int. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Choudhury, S.; Islam, N.; Sarkar, M.D.; Ali, M.A. Growth and Yield of Summer Tomato as Influenced by Plant Growth Regulators. Int. J. Sustain. Agric. 2013, 5, 25–28. [Google Scholar] [CrossRef]











| Experimental Variants | Commercial Name | Active Ingredient (According to Label) | Dosage | Observations |
|---|---|---|---|---|
| T1 (control) | - | - | - | - |
| T2 | Bonzi (Syngenta, Ontario, USA) | paclobutrazol (PBZ) | 30 mg/L PBZ | used two times |
| T3 | Alar 85 (Arysta LifeScience, Tokyo, Japan) | daminozide (DAM) | 2500 mg/L DAM | used two times |
| T4 | Florgib (Fine Americas, California, USA) | gibberellic acid (GA) | 20 mg/L GA3 | used two times |
| T5 | Fascination (Nufarm, Illinois, USA) | gibberellins A4A7 + 6-benzylaminopurine (GA4+7 + BAP) | 10 mg/L GA4+7 + 10 mg/L BAP | used two times |
| 15 November | 30 November | 14 December | 4 January | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rosette Diameter | Rosette Height | Rosette Diameter | Rosette Height | Number of Buds | Rosette Diameter | Rosette Height | Number of Buds | Rosette Diameter | Rosette Height | Number of Buds | Number of Flowers | |||||||||||||
| Mean | a | Mean | a | Mean | a | Mean | a | Mean | a | Mean | a | Mean | a | Mean | a | Mean | a | Mean | a | Mean | a | Mean | a | |
| Control | 19.48 | b | 8.27 | a | 21.33 | a | 8.58 | bc | 0.42 | ab | 22.77 | ab | 8.79 | b | 1.71 | b | 22.90 | b | 8.96 | c | 11.08 | b | 3.92 | ab |
| DAM | 19.68 | b | 8.16 | a | 21.25 | a | 8.42 | c | 0.58 | a | 22.38 | bc | 8.83 | b | 1.70 | bc | 22.79 | b | 9.38 | b | 8.58 | c | 3.25 | bc |
| PBZ | 19.98 | ab | 8.13 | a | 20.71 | b | 8.25 | c | 0.04 | c | 22.10 | c | 8.81 | b | 0.79 | c | 22.90 | c | 9.19 | bc | 7.08 | c | 2.29 | c |
| GA4+7 + BAP | 19.63 | b | 8.35 | a | 21.60 | a | 9.25 | a | 0.29 | abc | 23.02 | a | 9.48 | a | 2.83 | a | 23.74 | a | 10.33 | a | 23.09 | a | 5.13 | a |
| GA3 | 20.46 | a | 8.18 | a | 20.54 | b | 8.92 | b | 0.25 | bc | 21.02 | d | 8.90 | b | 2.67 | ab | 21.71 | d | 9.08 | bc | 25.22 | a | 5.13 | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bálint, J.; Benedek, K.; Csorba, A.B. Assessing the Effect of Plant Growth Stimulants and Retardants on Cyclamen “Halios F1 Salmon Rose” Cultivar. Horticulturae 2024, 10, 53. https://doi.org/10.3390/horticulturae10010053
Bálint J, Benedek K, Csorba AB. Assessing the Effect of Plant Growth Stimulants and Retardants on Cyclamen “Halios F1 Salmon Rose” Cultivar. Horticulturae. 2024; 10(1):53. https://doi.org/10.3390/horticulturae10010053
Chicago/Turabian StyleBálint, János, Klára Benedek, and Artúr Botond Csorba. 2024. "Assessing the Effect of Plant Growth Stimulants and Retardants on Cyclamen “Halios F1 Salmon Rose” Cultivar" Horticulturae 10, no. 1: 53. https://doi.org/10.3390/horticulturae10010053