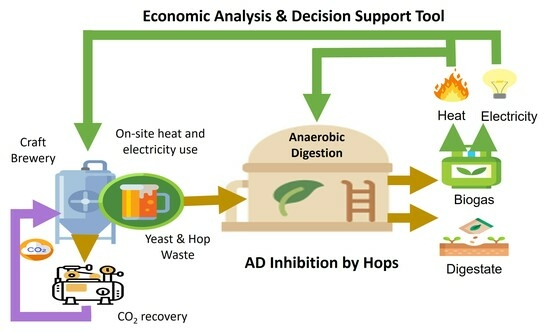
1. Introduction
Interest in craft beers is increasing worldwide due to their flavor, variety, and artisanal approach to brewing. Craft breweries are typically defined as those with an annual production of 0.7 million m
3 (6 million barrels) of beer or less [
1,
2]. Craft breweries have high water, energy and carbon dioxide (CO
2) demands, and generate large quantities of solid and liquid wastes and greenhouse gases. Spent grains account for up to 85% of the solid waste generated in craft breweries [
3] and are typically sent to farmers for use as animal feed. Beer brewing requires 4 to 20 m
3 of water to produce each m
3 of beer. Wastewater is generated from various processes, including low-strength wastewater from cleaning operations and high-strength wastewater, including trub, spent yeast, and hops. Spent yeast, which makes up the largest fraction of high-strength liquid waste, has high chemical oxygen demand (COD) concentrations ranging from 100,000 to 300,000 mg/L [
4]. While some of the yeast can be recycled within the brewery or directed for use as animal feed, most craft breweries direct this wastewater to local treatment plants, which often impose high waste surcharges [
5,
6]. Craft beer brewing is also energy intensive, with approximately 240 to 280 kWh of thermal energy and 75 to 138 kWh of electrical energy consumed per m
3 of beer produced [
7,
8].
Anaerobic digestion (AD) is a biological process that converts organic wastes into biogas, which is a mixture of methane (CH
4) and CO
2. Biogas can be further processed into renewable natural gas (RNG) and used onsite to meet a brewery’s thermal energy needs or processed into compressed natural gas (CNG) and liquefied natural gas (LNG) for offsite use. Alternatively, it can be utilized for generating electricity and heat through combined heat and power (CHP) systems to offset a brewery’s electrical and thermal demand [
9]. Many large breweries employ AD for both wastewater treatment and energy cost reduction [
10]. For example, Sierra Nevada Brewing Company (Chico, CA, USA) reported annual energy and waste management savings of >USD 500,000 after implementing AD [
11].
Beers containing large quantities of hops (
Humulus lupulus), such as India Pale Ales (IPAs), are a trademark of craft brewing. Spent hops have a bitter flavor and a lower nutritional value than spent grain. Hence, only a small portion of hop waste can be directed to animal feed [
4]. In addition, hop metabolites include alpha acids, beta acids, and Xanthohumol, which have antimicrobial properties that aid in beer preservation [
12,
13]. These compounds have been shown to inhibit CH
4 production in ruminant animals, which has been proposed as a way to increase the nutritive value of feeds while reducing greenhouse gas emissions from cattle [
14,
15,
16]. Two mechanisms have been identified for CH
4 inhibition in ruminants: (a) inhibition of Gram-positive bacteria in the acetogenic and acidogenic stage [
17,
18] and (b) inhibition of methanogenic archaea [
16].
Although it is evident that hop metabolites inhibit CH
4 production in cattle, the effect of hop metabolites on the AD of brewery waste has not previously been investigated. Sosa-Hernandez and colleagues conducted biomethane potential (BMP) assays with spent yeast from different sources and reported low CH
4 yields from hoppy beers (28 mLCH
4/gCOD) compared with less hoppy beers (42 and 68 mLCH
4/gCOD), suggesting potential inhibition by hop metabolites [
19].
Carbon dioxide (CO
2) is a by-product of beer fermentation and is also used in the brewing process for bottling, flushing, and carbonation. Prior studies have shown that CO
2 can be recovered from fermentation, scrubbed, and compressed for in-process recycling and reducing costs and greenhouse gas emissions [
20]. CO
2 that is recovered from fermenters is also a high-quality product without industrial contaminants that may be present when by-product CO
2 is purchased from ammonia and urea facilities. Recovered CO
2 can be further processed into dry ice and compressed or liquefied CO
2 for offsite applications. CO
2 recovery units are available as modular skid-mounted systems [
21]. Considering its economic and environmental benefits, CO
2 recovery could improve the sustainability of small craft breweries.
Several spreadsheet tools have been developed to aid in the economic and environmental assessment of AD systems. However, most of these tools focus on livestock manure as the primary AD substrate. For example, the US Environmental Protection Agency (US EPA) has developed a Co-Digestion Economic Analysis Tool (CoEAT) to evaluate the economic feasibility of AD co-digestion of manure with food waste, fats, oils, and grease [
22]. Astill and colleagues developed a tool to aid farmers in AD adoption decision-making. The tool is designed to assess the economic feasibility of AD using farm-derived feedstocks, including manure and crop residues [
23]. Therefore, the existing tools are not directly applicable to craft brewery waste. Furthermore, no prior study examines the economic tradeoffs of CO
2 recovery systems for small craft breweries.
The overall goal of this study was to improve the environmental and economic sustainability of small craft breweries by recovering bioenergy and CO2 for onsite use. The specific objectives were to: (1) investigate the effect of hops on spent yeast waste AD through BMP assays, (2) conduct bench-scale anaerobic sequencing batch reactor (ASBR) studies without and with hops addition to provide data for full-scale economic analysis, and (3) develop a tool to evaluate the feasibility of bioenergy and CO2 recovery at craft breweries depending on factors such as production volume, location, waste surcharges, CO2 costs, energy costs, and hop waste addition.
4. Conclusions
This study evaluated the effects of AD of spent brewery yeast, co-digestion of spent yeast with hops, and the economic feasibility of AD and CO2 recovery systems at craft breweries. Bench-scale experiments showed that the AD of yeast alone requires dilution with lower-strength waste, such as wastewater from cleaning operations, to avoid reactor overload since yeast has an acidic pH and high concentrations of readily bioavailable COD. During co-digestion, a 20% hop dosage resulted in little to no inhibition of methanogenesis, whereas a 40% hop dosage led to significantly lower methane yields. Future studies should consider pilot-scale AD studies with varying hop dosages.
An economic analysis tool was used to evaluate the feasibility of bioenergy and CO2 recovery at craft breweries. The findings indicated that AD and CO2 recovery were economically viable for breweries producing over 50,000 barrels annually. The analysis demonstrated that the AD + RNG option is more financially viable than the AD + CHP option. Implementation of CO2 recovery significantly reduced payback periods for AD plants. Although co-digestion with 20% hops waste led to a slight decrease in methane yield, it did not significantly impact the economic feasibility of the AD plant. Future studies should explore the economics of other pathways for resource recovery from craft breweries, including CO2 recovery from biogas and production of compressed natural gas (CNG), liquefied natural gas (LNG), dry ice, and compressed or liquefied CO2. In addition, the Excel tool should be compared with results from real-world breweries at different scales to enhance its usability.