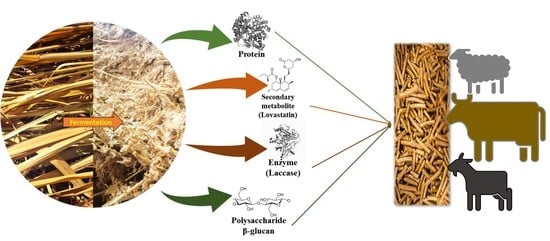
Nutraceutical Enrichment of Animal Feed by Filamentous Fungi Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Biomasses
2.2. Substrate Preparation, Fungi Cultivation, and Growth Measurement
2.3. Laccase Activity
2.4. Protein Quantification
2.5. β-Glucan Quantification
2.6. Lovastatin Quantification
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fungi Growth Rate
3.2. Laccase Activity
3.3. Total Protein and β-Glucan Content
3.4. Lovastatin Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucchese-Cheung, T.; de Aguiar, L.K.; de Lima, L.C.; Spers, E.E.; Quevedo-Silva, F.; Alves, F.V.; Giolo de Almeida, R. Brazilian Carbon Neutral Beef as an Innovative Product: Consumption Perspectives Based on Intentions’ Framework. J. Food Prod. Mark. 2021, 27, 384–398. [Google Scholar] [CrossRef]
- Feltran-Barbieri, R.; Féres, J.G. Degraded Pastures in Brazil: Improving Livestock Production and Forest Restoration. R. Soc. Open Sci. 2021, 8, 201854. [Google Scholar] [CrossRef] [PubMed]
- Salton, J.C.; Mercante, F.M.; Tomazi, M.; Zanatta, J.A.; Concenço, G.; Silva, W.M.; Retore, M. Integrated Crop-Livestock System in Tropical Brazil: Toward a Sustainable Production System. Agric. Ecosyst. Environ. 2014, 190, 70–79. [Google Scholar] [CrossRef]
- D’aurea, A.P.; da Silva Cardoso, A.; Guimarães, Y.S.R.; Fernandes, L.B.; Ferreira, L.E.; Reis, R.A. Mitigating Greenhouse Gas Emissions from Beef Cattle Production in Brazil through Animal Management. Sustainability 2021, 13, 7207. [Google Scholar] [CrossRef]
- Nur-Nazratul, F.M.Y.; Rakib, M.R.M.; Zailan, M.Z.; Yaakub, H. Enhancing in Vitro Ruminal Digestibility of Oil Palm Empty Fruit Bunch by Biological Pre-Treatment with Ganoderma Lucidum Fungal Culture. PLoS ONE 2021, 16, e0258065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Y.; Luo, L.; Zhang, H.; Liao, Y.; Gou, C. Enhancement of the Nutritional Value of Fermented Corn Stover as Ruminant Feed Using the Fungi Pleurotus spp. Sci. Rep. 2021, 11, 11961. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Lu, N.; Liu, Y.; Zhang, S.; Jiang, H.; Wang, H.; Wang, W.; Li, S. Aspergillus Oryzae and Aspergillus Niger Co-Cultivation Extract Affects in Vitro Degradation, Fermentation Characteristics, and Bacterial Composition in a Diet-Specific Manner. Animals 2021, 11, 1248. [Google Scholar] [CrossRef]
- Lubis, D.; Wina, E.; Haryanto, B.; Suhargiyantatmo, T. Feeding of Aspergillus Oryzae Fermentation Culture (AOFC) to Growing Sheep: 2. Growth Rate and Feed Efficiency. J. Ilmu Ternak Vet. 2002, 7, 214–219. [Google Scholar]
- Ábrego-Gacía, A.; Poggi-Varaldo, H.M.; Mendoza-Vargas, A.; Mercado-Valle, F.G.; Ríos-Leal, E.; Ponce-Noyola, T.; Calva-Calva, G. Effects of Fermented Oat Straw as a Lovastatin Carrier on in Vitro Methane Production and Rumen Microbiota. Front. Energy Res. 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Zabek, K.; Milewski, S.; Wójcik, R.; Siwicki, A.K. Effect of β-1,3/1,6-D-Glucan in Diet on Productivity and Humoral and Cellular Defense Mechanisms in Sheep. Acta Vet. Brno 2013, 82, 141–146. [Google Scholar] [CrossRef]
- Ballou, M.A.; Davis, E.M.; Kasl, B.A. Nutraceuticals an Alternative Strategy for the Use of Antimicrobials. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 507–534. [Google Scholar] [CrossRef] [PubMed]
- Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Prospects and Feasibility of Fungal Pretreatment of Agricultural Biomass for Ruminant Feeding. Anim. Feed Sci. Technol. 2020, 268, 114577. [Google Scholar] [CrossRef]
- Kumar, V.P.; Rao, R.G.; Dhali, A.; Thammiaha, V.; Sridhar, M. Evaluation of Exogenous Laccase Enzyme Treated Finger Millet Straw on Body Weight Gain, DM Intake and Nutrient Digestibility in Heifers. Indian J. Anim. Res. 2021, 55, 457–462. [Google Scholar] [CrossRef]
- Lucio, B.S.V.-D.; Domínguez, E.M.H.; García, M.V.; Díaz-Godínez, G.; Mandujano-Gonzalez, V.; Mendoza-Mendoza, B.; Álvarez-Cervantes, J. Exogenous Enzymes as Zootechnical Additives in Animal Feed: A Review. Catalysts 2021, 11, 851. [Google Scholar] [CrossRef]
- Yue, Z.Q.; Xu, Y.Z.; Wang, C.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Chen, L.; Pei, C.X.; Zhang, Y.L.; et al. Effects of Dietary Laccase Supplementation on Growth Performance, Nutrient Digestion, Rumen Fermentation and Microbiota in Dairy Bulls. Anim. Feed Sci. Technol. 2020, 269, 114645. [Google Scholar] [CrossRef]
- Tao, M.A.; Yan, T.U.; Zhang, N.F.; Guo, J.P.; Deng, K.D.; Yi, Z.H.O.U.; Qiang, Y.U.N.; Diao, Q.Y. Effects of Dietary Yeast β-Glucan on Nutrient Digestibility and Serum Profiles in Pre-Ruminant Holstein Calves. J. Integr. Agric. 2015, 14, 749–757. [Google Scholar] [CrossRef]
- Saadei Khalkhane, A.; Habibian, R. Effect of Dietary B-Glucan Supplementation on Humoral and Cellular Immunologic Factors in Lambs. Glob. Vet. 2013, 11, 38–43. [Google Scholar] [CrossRef]
- Kruger, D.; Werf, M. Van der Benefits of Application of Yeast β-Glucansruminants; Ohly GmbH: Hamburg, Germany, 2018. [Google Scholar]
- Jahromi, M.F.; Liang, J.B.; Mohamad, R.; Goh, Y.M.; Shokryazdan, P.; Ho, Y.W. Lovastatin-Enriched Rice Straw Enhances Biomass Quality and Suppresses Ruminal Methanogenesis. BioMed Res. Int. 2013, 2013, 397934. [Google Scholar] [CrossRef]
- Neto, C.B.S.; Conceição, A.A.; Gomes, T.G.; de Aquino Ribeiro, J.A.; Campanha, R.B.; Barroso, P.A.V.; Machado, A.E.V.; Mendonça, S.; De Siqueira, F.G.; Miller, R.N.G. A Comparison of Physical, Chemical, Biological and Combined Treatments for Detoxification of Free Gossypol in Crushed Whole Cottonseed. Waste Biomass Valoriz. 2021, 12, 3965–3975. [Google Scholar] [CrossRef]
- Guimarães, M.B.; de Siqueira, F.G.; Campanha, R.B.; de Aquino Ribeiro, J.A.; Oliveira Magalhães, P.; Mendonça, S. Evaluation of Bio-Detoxification of Jatropha Curcas Seed Cake and Cottonseed Cake by Basidiomycetes: Nutritional and Antioxidant Effects. Waste Biomass Valoriz. 2022, 13, 1475–1490. [Google Scholar] [CrossRef]
- Su, B.; Chen, X. Current Status and Potential of Moringa Oleifera Leaf as an Alternative Protein Source for Animal Feeds. Front. Vet. Sci. 2020, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Heinzkill, M.; Bech, L.; Halkier, T.; Schneider, P.; Anke, T. Characterization of Laccases and Peroxidases from Wood-Rotting Fungi (Family Coprinaceae). Appl. Environ. Microbiol. 1998, 64, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Wolfenden, B.S.; Willson, R. Radical-Cations as Reference Chromogens in Kinetic Studies of Ono-Electron Transfer Reactions: Pulse Radiolysis Studies of 2,2′-Azinobis-(3-Ethylbenzthiazoline-6-Sulphonate). J. Chem. Soc. Perkin Trans. 1982, 2, 805–812. [Google Scholar] [CrossRef]
- Chebaibi, S.; Leriche Grandchamp, M.; Burgé, G.; Clément, T.; Allais, F.; Laziri, F. Improvement of Protein Content and Decrease of Anti-Nutritional Factors in Olive Cake by Solid-State Fermentation: A Way to Valorize This Industrial by-Product in Animal Feed. J. Biosci. Bioeng. 2019, 128, 384–390. [Google Scholar] [CrossRef]
- Krotz, L. Elemental Analysis: N/Protein and CHNS Determination of Insect-Based Food and Animal Feed by Dumas Method; Thermo Fisher Scientific: Milan, Italy, 2019. [Google Scholar]
- Barbarino, E.; Lourenço, S.O. Comparison of CHN Analysis and Hach Acid Digestion to Quantify Total Nitrogen in Marine Organisms. Limnol. Oceanogr. Methods 2009, 7, 751–760. [Google Scholar] [CrossRef]
- Megazyme. Mushroom and Yeast Beta-Glucan Assay Procedure (K-YBGL 02/21); Megazyme: Wicklow, Ireland, 2021; Volume 21. [Google Scholar]
- Wang, J.; Luzum, J.A.; Phelps, M.A.; Kitzmiller, J.P. Liquid Chromatography—Tandem Mass Spectrometry Assay for the Simultaneous Quantification of Simvastatin, Lovastatin, Atorvastatin, and Their Major Metabolites in Human Plasma. J. Chromatogr. B 2015, 983, 18–25. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, F.; Fang, Y.; Zhou, D.; Wang, S.; Wu, D.; Wang, L.; Zhong, R. High-Potency White-Rot Fungal Strains and Duration of Fermentation to Optimize Corn Straw as Ruminant Feed. Bioresour. Technol. 2020, 312, 67–78. [Google Scholar] [CrossRef]
- Sharma, R.; Kocher, G.S.; Rao, S.S.; Oberoi, H.S. Improved Production of Multi-Component Cellulolytic Enzymes Using Sweet Sorghum Bagasse and Thermophilic Aspergillus terreus RWY Through Statistical Process Optimization. Waste Biomass Valoriz. 2020, 11, 3355–3369. [Google Scholar] [CrossRef]
- Tuyen, V.D.; Cone, J.W.; Baars, J.J.P.; Sonnenberg, A.S.M.; Hendriks, W.H. Fungal Strain and Incubation Period Affect Chemical Composition and Nutrient Availability of Wheat Straw for Rumen Fermentation. Bioresour. Technol. 2012, 111, 336–342. [Google Scholar] [CrossRef]
- Shrivastava, B.; Jain, K.K.; Kalra, A.; Kuhad, R.C. Bioprocessing of Wheat Straw into Nutritionally Rich and Digested Cattle Feed. Sci. Rep. 2014, 4, 6360. [Google Scholar] [CrossRef]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The Impact of Mushroom Polysaccharides on Gut Microbiota and Its Beneficial Effects to Host: A Review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Alzaban, M.I.; Albarakaty, F.M.; El-aziz, A.R.M.A.; Alrokban, A.H.; Mahmoud, M.A. Transcriptome Profiling Reveals Differential Gene Expression of Laccase Genes in Aspergillus terreus KC462061 during Biodegradation of Crude Oil. Biology 2022, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.A.; Cabreras, C.E.V.; Torta, L.; Laudicina, A.; Mirabile, G. Ligninolytic Potential of Curvularia Kusanoi L7 Laccases for Animal Production. Cuba. J. Agric. Sci. 2020, 54, 157–167. [Google Scholar]
- Beauchemin, K.A.; Colombatto, D.; Morgavi, D.P.; Yang, W.Z.; Rode, L.M. Mode of Action of Exogenous Cell Wall Degrading Enzymes for Ruminants. Can. J. Anim. Sci. 2003, 84, 22. [Google Scholar] [CrossRef]
- Peláez, R.D.R.; Wischral, D.; Mendes, T.D.; Pacheco, T.F.; Urben, A.F.; Helm, C.V.; Mendonça, S.; Balan, V.; de Siqueira, F.G. Co-Culturing of Micro- and Macro-Fungi for Producing Highly Active Enzyme Cocktail for Producing Biofuels. Bioresour. Technol. Rep. 2021, 16, 100833. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Jokharidze, T.; Kobakhidze, A.; Elisashvili, V. Enhancement of Laccase Production by Cerrena Unicolor through Fungal Interspecies Interaction and Optimum Conditions Determination. Arch. Microbiol. 2021, 203, 3905–3917. [Google Scholar] [CrossRef] [PubMed]
- Lallawmsanga; Leo, V.V.; Passari, A.K.; Muniraj, I.K.; Uthandi, S.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Singh, B.P. Elevated Levels of Laccase Synthesis by Pleurotus Pulmonarius BPSM10 and Its Potential as a Dye Decolorizing Agent. Saudi J. Biol. Sci. 2019, 26, 464–468. [Google Scholar] [CrossRef]
- Kirst Tychanowicz, G.; De Souza, D.F.; Souza, C.G.M.; Kadowaki, M.K.; Peralta, R.M. Copper Improves the Production of Laccase by the White- Rot Fungus Pleurotus Pulmonarius in Solid State Fermentation. Braz. Arch. Biol. Technol. 2006, 49, 699–704. [Google Scholar] [CrossRef]
- De Souza, D.F.; Tychanowicz, G.K.; De Souza, C.G.M.; Peralta, R.M. Co-Production of Ligninolytic Enzymes by Pleurotus Pulmonarius on Wheat Bran Solid State Cultures. J. Basic Microbiol. 2006, 46, 126–134. [Google Scholar] [CrossRef]
- Floudas, D.; Held, B.W.; Riley, R.; Nagy, L.G.; Koehler, G.; Ransdell, A.S.; Younus, H.; Chow, J.; Chiniquy, J.; Lipzen, A.; et al. Evolution of Novel Wood Decay Mechanisms in Agaricales Revealed by the Genome Sequences of Fistulina Hepatica and Cylindrobasidium Torrendii. Fungal Genet. Biol. 2015, 76, 78–92. [Google Scholar] [CrossRef]
- Peláez, F.; Martínez, M.J.; Martínez, A.T. Screening of 68 Species of Basidiomycetes for Enzymes Involved in Lignin Degradation. Mycol. Res. 1995, 99, 37–42. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhang, J.L.; Hu, K.H.; Zhang, W.G. The Relationship between Lignin Peroxidase and Manganese Peroxidase Production Capacities and Cultivation Periods of Mushrooms. Microb. Biotechnol. 2013, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, F.W.M.R.; Baum, S.; Fink, S. Dual Modes of Degradation by Fistulina Hepatica in Xylem Cell Walls of Quercus Robur. Mycol. Res. 2000, 104, 846–852. [Google Scholar] [CrossRef]
- Yuliana, T.; Putri, N.Z.; Komara, D.Z.; Mardawati, E.; Lanti, I.; Rahimah, S. Study of Ganoderma Lucidum in Laccase Production Using Corncob and Paddies Straw Substrates on Submerged Fermentation System. Pakistan J. Biol. Sci. 2020, 23, 1060–1065. [Google Scholar] [CrossRef]
- Manavalan, T.; Manavalan, A.; Thangavelu, K.P.; Heese, K. Characterization of Optimized Production, Purification and Application of Laccase from Ganoderma Lucidum. Biochem. Eng. J. 2013, 70, 106–114. [Google Scholar] [CrossRef]
- Rodrigues, E.M.; Karp, S.G.; Malucelli, L.C.; Helm, C.V.; Alvarez, T.M. Evaluation of Laccase Production by Ganoderma Lucidum in Submerged and Solid-State Fermentation Using Different Inducers. J. Basic Microbiol. 2019, 59, 784–791. [Google Scholar] [CrossRef]
- Shaikh, S.; Shaikh, I.; Dixit, P. Production and Characterization of Laccase from Aspergillus terreus Isolated from Saw Mill Soil of Osmanabad. Int. J. Adv. Res. 2020, 8, 626–632. [Google Scholar] [CrossRef]
- Stoffel, F.; de Santana, W.O.; Gregolon, J.G.N.; Kist, T.B.L.; Fontana, R.C.; Camassola, M. Production of Edible Mycoprotein Using Agroindustrial Wastes: Influence on Nutritional, Chemical and Biological Properties. Innov. Food Sci. Emerg. Technol. 2019, 58, 102227. [Google Scholar] [CrossRef]
- Ellamar, J.B.; Valdez, M.T.S.; Inalvez, A.E.; Maniquez, M.C.; Rodriguez, J.M.; Tarozza, D.B. Bioconversion of Lignocellulosic Agricultural By-Products by Microorganisms into High Mycoprotein Feeds. Philipp. J. Vet. Anim. Sci. 2019, 45, 75–86. [Google Scholar]
- Khonkhaeng, B.; Cherdthong, A. Improving Nutritive Value of Purple Field Corn Residue and Rice Straw by Culturing with White-Rot Fungi. J. Fungi 2020, 6, 69. [Google Scholar] [CrossRef]
- Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Variation in the Solubilization of Crude Protein in Wheat Straw by Different White-Rot Fungi. Anim. Feed Sci. Technol. 2018, 242, 135–143. [Google Scholar] [CrossRef]
- Karimi, S.; Soofiani, N.M.; Mahboubi, A.; Ferreira, J.A.; Lundh, T.; Kiessling, A.; Taherzadeh, M.J. Evaluation of Nutritional Composition of Pure Filamentous Fungal Biomass as a Novel Ingredient for Fish Feed. Fermentation 2021, 7, 152. [Google Scholar] [CrossRef]
- Ekute, B. Nutritional Profile of Two Nigerian Edible Mushrooms: Pleurotus Ostreatus and Pleurotus Pulmonarius. J. Appl. Sci. Environ. Manag. 2018, 22, 1745–1747. [Google Scholar] [CrossRef]
- Oyetayo, V.O.; Ogidi, C.O.; Bayode, S.O.; Enikanselu, F.F. Evaluation of Biological Efficiency, Nutrient Contents and Antioxidant Activity of Pleurotus Pulmonarius Enriched with Zinc and Iron. Indian Phytopathol. 2021, 74, 901–910. [Google Scholar] [CrossRef]
- Inácio, F.D.; Ferreira, R.O.; de Araujo, C.A.V.; Peralta, R.M.; de Souza, C.G.M. Production of Enzymes and Biotransformation of Orange Waste by Oyster Mushroom, Pleurotus pulmonarius (Fr.). Adv. Microbiol. 2015, 5, 52997. [Google Scholar] [CrossRef]
- Akinfemi, A.; Adu, O.A.; Doherty, F. Conversion of Sorghum Stover into Animal Feed with White-Rot Fungi: Pleurotus Ostreatus and Pleurotus Pulmonarius. Afr. J. Biotechnol. 2010, 9, 1706–1712. [Google Scholar] [CrossRef]
- Akinfemi, A.; Ogunwole, O.A. ChemiCal Composition and in Vitro Digestibility of RiCe Straw Treated with Pleurotus Ostreatus, Pleurotus Pulmonarius and Pleurotus Tuber-Regium. Slovak J. Anim. Sci. 2012, 45, 14–20. [Google Scholar]
- Montañez-Valdez, O.D.; Flores, E.O.G.; García, J.A.M.; Chavira, J.S.; Rubio, R.R.; Ortiz, J.J.G.P. Use of Pleurotus Pulmonarius to Change the Nutritional Quality of Wheat Straw. I. Effect on Chemical Composition. Interciencia 2008, 33, 435–438. [Google Scholar]
- Martinez, Y.; Paredes, J.; Avellaneda, M.; Botello, A.; Valdivie, M. Diets with Ganoderma Lucidum Mushroom Powder and Zinc-Bacitracin on Growth Performance, Carcass Traits, Lymphoid Organ Weights and Intestinal Characteristics in Broilers. Brazilian J. Poult. Sci. 2022, 24, 1–12. [Google Scholar] [CrossRef]
- Chen, S.D.; Hsieh, M.C.; Chiou, M.T.; Lai, Y.S.; Cheng, Y.H. Effects of Fermentation Products of Ganoderma Lucidum on Growth Performance and Immunocompetence in Weanling Pigs. Arch. Anim. Nutr. 2008, 62, 22–32. [Google Scholar] [CrossRef]
- Misra, A.K.; Mishra, A.S.; Tripathi, M.K.; Prasad, R.; Vaithiyanathan, S.; Jakhmola, R.C. Optimization of Solid State Fermentation of Mustard (Brassica Campestris) Straw for Production of Animal Feed by White Rot Fungi (Ganoderma lucidum). Asian-Australas. J. Anim. Sci. 2007, 20, 208–213. [Google Scholar] [CrossRef]
- Han, J.R.; An, C.H.; Yuan, J.M. Solid-State Fermentation of Cornmeal with the Basidiomycete Ganoderma Lucidum for Degrading Starch and Upgrading Nutritional Value. J. Appl. Microbiol. 2005, 99, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Jaganmohan, B.; Daas, B.P.; Prasad, S.V. Production of Single Cell Protein (SCP) with Aspergillus terreus Using Solid State Fermentation. Eur. J. Biol. Sci. 2013, 5, 38–43. [Google Scholar] [CrossRef]
- Shahzad, M.A.; Rajoka, M.I. Single Cell Protein Production from Aspergillus terreus and Its Evaluation in Broiler Chick. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 137–141. [Google Scholar] [CrossRef]
- Garg, S.K.; Neelakantan, S. Production of SCP and Cellulase by Aspergillus terreus from Bagasse Substrate. Biotechnol. Bioeng. 1982, 24, 2407–2417. [Google Scholar] [CrossRef]
- Miller, T.F.; Srinivasan, V.R. Producton of Single-cell Protein from Cellulose by Aspergillus terreus. Biotechnol. Bioeng. 1983, 25, 1509–1519. [Google Scholar] [CrossRef]
- Ouzouni, P.K.; Petridis, D.; Koller, W.D.; Riganakos, K.A. Nutritional Value and Metal Content of Wild Edible Mushrooms Collected from West Macedonia and Epirus, Greece. Food Chem. 2009, 115, 1575–1580. [Google Scholar] [CrossRef]
- Cortina-escribano, M.; Pihlava, J.; Miina, J.; Veteli, P.; Linnakoski, R.; Vanhanen, H. Effect of Strain, Wood Substrate and Cold Treatment on the Yield and β -Glucan Content of Ganoderma Lucidum Fruiting Bodies. Molecules 2020, 25, 4732. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of Beta-Glucan Contents in Commercially Cultivated and Wild Growing Mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Hamzah, N.S.A. Keong Screening and Characterization of Endopolysaccharides from Pleurotus Pulmonarius in Submerged Culture Fermentation. J. Sains Nukl. Malays. 2017, 29, 52–59. [Google Scholar]
- Contato, A.G.; Inácio, F.D.; de Araújo, C.A.V.; Brugnari, T.; Maciel, G.M.; Haminiuk, C.W.I.; Bracht, A.; Peralta, R.M.; de Souza, C.G.M. Comparison between the Aqueous Extracts of Mycelium and Basidioma of the Edible Mushroom Pleurotus Pulmonarius: Chemical Composition and Antioxidant Analysis. J. Food Meas. Charact. 2020, 14, 830–837. [Google Scholar] [CrossRef]
- Atoji-Henrique, K.; Henrique, D.S.; Glória, L.S.; Mazaro, S.M.; Casagrande, M. Influence of Substrate Composition on Beta-Glucans Production and Growth of Ganoderma Lucidum. J. Agric. Sci. 2017, 9, 190. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.A.; Pletsa, V.; Kyriacou, A.; Zervakis, G.I. Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma Lucidum and Pleurotus Ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef]
- Tsubaki, K. Betaglucan-Containing Fat and Oil Compositions and Novel Microorganism Capable of Producing Beta-Glucan [EP1533382A1]. U.S. Patent 7,442,541, 28 October 2008. [Google Scholar]
- Wang, C.; Mao, W.; Chen, Z.; Zhu, W.; Chen, Y.; Zhao, C.; Li, N.; Yan, M.; Liu, X.; Guo, T. Purification, Structural Characterization and Antioxidant Property of an Extracellular Polysaccharide from Aspergillus terreus. Process Biochem. 2013, 48, 1395–1401. [Google Scholar] [CrossRef]
- Costa, C.R.L.d.M.; Menolli, R.A.; Osaku, E.F.; Tramontina, R.; de Melo, R.H.; do Amaral, A.E.; Duarte, P.A.D.; de Carvalho, M.M.; Smiderle, F.R.; Silva, J.L.d.C.; et al. Exopolysaccharides from Aspergillus terreus: Production, Chemical Elucidation and Immunoactivity. Int. J. Biol. Macromol. 2019, 139, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, B.; Saravanan, T. Optimization of B-Glucan Production from Lower Fungi Using Central Composite Design and Its Biological Application. Int. J. Comput. Appl. 2012, 49, 23–28. [Google Scholar] [CrossRef]
- Uchiyama, H.; Iwai, A.; Asada, Y.; Muramatsu, D.; Aoki, S.; Kawata, K.; Kusano, K.; Nagashima, K.; Yasokawa, D.; Okabe, M.; et al. A Small Scale Study on the Effects of Oral Administration of the β-Glucan Produced by Aureobasidium pullulans on Milk Quality and Cytokine Expressions of Holstein Cows, and on Bacterial Flora in the Intestines of Japanese Black Calves. BMC Res. Notes 2012, 5, 189. [Google Scholar] [CrossRef]
- Wójcik, R. The Effect of Leiber Beta-S (1,3-1,6-β-D-Glucan) on the Phagocytic Activity and Oxidative Metabolism of Peripheral Blood Granulocytes and Monocytes in Calves. Acta Vet. Brno 2014, 83, 347–354. [Google Scholar] [CrossRef]
- Cherdthong, A.; Seankamsorn, A.; Suriyapha, C.; Chanjula, P.; Wanapat, M. Effect of Beta-Glucan Supplementation on Feed Intake, Digestibility of Nutrients and Ruminal Fermentation in Thai Native Beef Cattle. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1509–1514. [Google Scholar] [CrossRef]
- Wang, L.; Xia, W.H.; Wang, J.H.; Fan, R.F.; Niu, X.D.; Wang, Y.M.; Li, Q.L.; Wang, Z.Y.; Wang, Z.H. Effects of Beta-1,3-Glucan Supplementation on Concentrations of Serum Metabolites in Transition Holstein Cows. Res. Vet. Sci. 2020, 132, 250–256. [Google Scholar] [CrossRef]
- Raa, J. Immune Modulation by Non-Digestible and Non-Absorbable Beta-1,3/1,6-Glucan. Microb. Ecol. Health Dis. 2015, 26, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Grove, A.V.; Kaiser, C.R.; Iversen, N.; Hafla, A.; Robison, B.L.; Bowman, J.G.P. Digestibility of Barley Beta-Glucan in Cattle. Proc. West. Sect. Am. Soc. Anim. Sci. 2006, 57, 367–369. [Google Scholar]
- Lan, W.; Yang, C. Ruminal Methane Production: Associated Microorganisms and the Potential of Applying Hydrogen-Utilizing Bacteria for Mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Demonfort Nkamga, V.; Armstrong, N.; Drancourt, M. In Vitro Susceptibility of Cultured Human Methanogens to Lovastatin. Int. J. Antimicrob. Agents 2017, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.F.; Liang, J.B.; Ho, Y.W.; Mohamad, R.; Goh, Y.M.; Shokryazdan, P. Lovastatin Production by Aspergillus terreus Using Agro-Biomass as Substrate in Solid State Fermentation. J. Biomed. Biotechnol. 2012, 2012, 196264. [Google Scholar] [CrossRef]
- Wei, P.; Xu, Z.; Cen, P. Lovastatin Production by Aspergillus terreus in Solid State and Submerged Fermentations. J. Zhejiang Univ. Sci. A 2007, 8, 1521–1526. [Google Scholar] [CrossRef]
- Patil, R.H.; Krishnan, P.; Maheshwari, V.L. Production of Lovastatin by Wild Strains of Aspergillus terreus. Nat. Prod. Commun. 2011, 6, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.H.A.; Hasan, H.; Montoya, A.; Abbas, A. Lovastatin and (+)-Geodin Production by Aspergillus terreus from Crude Glycerol. Eng. Life Sci. 2015, 15, 220–228. [Google Scholar] [CrossRef]
- Mohd Azlan, P.; Jahromi, M.F.; Ariff, M.O.; Ebrahimi, M.; Candyrine, S.C.L.; Liang, J.B. Aspergillus terreus Treated Rice Straw Suppresses Methane Production and Enhances Feed Digestibility in Goats. Trop. Anim. Health Prod. 2018, 50, 565–571. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Inhibition of Growth of Methane-Producing Bacteria of the Ruminant Forestomach by Hydroxymethylglutaryl~SCoA Reductase Inhibitors. J. Dairy Sci. 2001, 84, 1445–1448. [Google Scholar] [CrossRef]
- Lee, J.; Shi, Y.M.; Grün, P.; Gube, M.; Feldbrügge, M.; Bode, H.; Hennicke, F. Identification of Feldin, an Antifungal Polyyne from the Beefsteak Fungus Fistulina Hepatica. Biomolecules 2020, 10, 1502. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Zhao, W.; Zhong, J.J. Biotechnological Production and Application of Ganoderic Acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Degenkolb, T.; Vilcinskas, A. Metabolites from Nematophagous Fungi and Nematicidal Natural Products from Fungi as Alternatives for Biological Control. Part II: Metabolites from Nematophagous Basidiomycetes and Non-Nematophagous Fungi. Appl. Microbiol. Biotechnol. 2016, 100, 3813–3824. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Reis, C.E.; Ogero D’Otaviano, L.; Rajendran, A.; Hu, B. Co-Culture of Filamentous Feed-Grade Fungi and Microalgae as an Alternative to Increase Feeding Value of Ethanol Coproducts. Fermentation 2018, 4, 86. [Google Scholar] [CrossRef]



| Plant Biomass | Nitrogen (%) |
|---|---|
| Corn grain | 0.93 ± 0.25 |
| Sorghum grain | 1.03 ± 0.03 |
| Oat grain | 1.82 ± 0.17 |
| Pigeon pea plant (leaves, branches, pods, and stems) | 1.60 ± 0.27 |
| Cottonseed cake | 2.15 ± 0.53 |
| Coast-cross | 0.32 ± 0.04 |
| Rice husk | 0.33 ± 0.09 |
| Moringa plant (leaves, branches, pods, and stems) | 0.90 ± 0.37 |
| Substrates | Starchy Grain | Lignin/Cellulose | Protein |
|---|---|---|---|
| ST-01 | Sorghum | - | - |
| ST-02 | - | CSC | |
| ST-03 | Coast-cross | - | |
| ST-04 | Coast-cross | CSC | |
| ST-05 | Rice husk | - | |
| ST-06 | Rice husk | CSC | |
| ST-07 | Moringa | - | |
| ST-08 | Moringa | CSC | |
| CT-01 | Corn | - | - |
| CT-02 | - | CSC | |
| CT-03 | Coast-cross | - | |
| CT-04 | Coast-cross | CSC | |
| CT-05 | Rice husk | - | |
| CT-06 | Rice husk | CSC | |
| CT-07 | Moringa | - | |
| CT-08 | Moringa | CSC | |
| OT-01 | Oat | - | - |
| OT-02 | - | CSC | |
| OT-03 | Coast-cross | - | |
| OT-04 | Coast-cross | CSC | |
| OT-05 | Rice husk | - | |
| OT-06 | Rice husk | CSC | |
| OT-07 | Moringa | - | |
| OT-08 | Moringa | CSC | |
| ST-09 | Sorghum | - | - |
| ST-10 | Coast-cross | Pigeon pea | |
| ST-11 | Rice husk | ||
| ST-12 | Moringa | ||
| CT-09 | Corn | - | - |
| CT-10 | Coast-cross | Pigeon pea | |
| CT-11 | Rice husk | ||
| CT-12 | Moringa | ||
| OT-09 | Oat | - | - |
| OT-10 | Coast-cross | Pigeon pea | |
| OT-11 | Rice husk | ||
| OT-12 | Moringa |
| Substrates | P. lecomtei BRM 044603 | G. lucidum BRM 055670 | F. hepatica BRM 047114 | P. pulmonarius BRM 055674 |
|---|---|---|---|---|
| ST-01 | 0.304 ± 0.070 | 0.287 ± 0.050 | 0.271 ± 0.088 | 0.346 ± 0.013 |
| ST-02 | 0.194 ± 0.112 | 0.191 ± 0.010 | 0.058 ± 0.053 F | 0.041 ± 0.062 F |
| ST-03 | 0.436 ± 0.084 | 0.458 ± 0.098 | 0.243 ± 0.110 | 0.487 ± 0.012 |
| ST-04 | 0.107 ± 0.098 | 0.000 ± 0.000 G | 0.183 ± 0.097 E | 0.097 ± 0.039 E |
| ST-05 | 0.275 ± 0.045 | 0.213 ± 0.128 | 0.173 ± 0.046 E | 0.293 ± 0.143 |
| ST-06 | 0.110 ± 0.096 E | 0.058 ± 0.101 F | 0.000 ± 0.000 G | 0.049 ± 0.017 F |
| ST-07 | 0.488 ± 0.024 | 0.342 ± 0.076 | 0.161 ± 0.029 E | 0.337 ± 0.199 |
| ST-08 | 0.000 ± 0.000 G | 0.051 ± 0.088 F | 0.211 ± 0.063 | 0.138 ± 0.076 E |
| ST-09 | 0.210 ± 0.006 | 0.275 ± 0.080 | 0.193 ± 0.080 | 0.307 ± 0.021 |
| ST-10 | 0.187 ± 0.052 E | 0.456 ± 0.029 | 0.252 ± 0.209 | 0.439 ± 0.014 |
| ST-11 | 0.439 ± 0.059 | 0.477 ± 0.004 | 0.376 ± 0.043 | 0.454 ± 0.023 |
| ST-12 | 0.422 ± 0.004 | 0.297 ± 0.174 | 0.156 ± 0.029 E | 0.254 ± 0.123 |
| CT-01 | 0.429 ± 0.040 | 0.052 ± 0.090 F | 0.000 ± 0.000 G | 0.141 ± 0.010 E |
| CT-02 | 0.205 ± 0.035 | 0.014 ± 0.025 F | 0.204 ± 0.096 | 0.099 ± 0.093 E |
| CT-03 | 0.330 ± 0.003 | 0.113 ± 0.195 E | 0.141 ± 0.071 E | 0.449 ± 0.038 |
| CT-04 | 0.235 ± 0.035 | 0.048 ± 0.084 F | 0.188 ± 0.075 E | 0.110 ± 0.066 E |
| CT-05 | 0.467 ± 0.021 | 0.000 ± 0.000 G | 0.261 ± 0.027 | 0.398 ± 0.011 |
| CT-06 | 0.235 ± 0.063 | 0.000 ± 0.000 G | 0.135 ± 0.047E | 0.102 ± 0.054 E |
| CT-07 | 0.211 ± 0.019 | 0.547 ± 0.042 ABCD | 0.351 ± 0.062 | 0.505 ± 0.088 |
| CT-08 | 0.270 ± 0.070 | 0.453 ± 0.093 | 0.195 ± 0.028 | 0.297 ± 0.090 |
| CT-09 | 0.349 ± 0.021 | 0.390 ± 0.077 | 0.398 ± 0.088 | 0.317 ± 0.115 |
| CT-10 | 0.391 ± 0.140 | 0.441 ± 0.058 | 0.274 ± 0.165 | 0.332 ± 0.004 |
| CT-11 | 0.428 ± 0.096 | 0.478 ± 0.014 | 0.226 ± 0.042 | 0.228 ± 0.170 |
| CT-12 | 0.365 ± 0.259 | 0.325 ± 0.190 | 0.270 ± 0.066 | 0.414 ± 0.020 |
| OT-01 | 0.366 ± 0.086 | 0.708 ± 0.035A | 0.284 ± 0.015 | 0.333 ± 0.034 |
| OT-02 | 0.364 ± 0.077 | 0.317 ± 0.120 | 0.220 ± 0.107 | 0.283 ± 0.121 |
| OT-03 | 0.466 ± 0.017 | 0.547 ± 0.147 ABCD | 0.576 ± 0.030 ABC | 0.442 ± 0.024 |
| OT-04 | 0.204 ± 0.138 | 0.270 ± 0.067 | 0.135 ± 0.016 E | 0.295 ± 0.001 |
| OT-05 | 0.398 ± 0.039 | 0.000 ± 0.000 G | 0.265 ± 0.027 | 0.166 ± 0.057 E |
| OT-06 | 0.129 ± 0.069 E | 0.000 ± 0.000 G | 0.125 ± 0.048 E | 0.100 ± 0.005 E |
| OT-07 | 0.398 ± 0.007 | 0.584 ± 0.017 AB | 0.551 ± 0.035 ABCD | 0.261 ± 0.113 |
| OT-08 | 0.000 ± 0.000 G | 0.171 ± 0.091 E | 0.059 ± 0.054 F | 0.148 ± 0.081 E |
| OT-09 | 0.407 ± 0.005 | 0.438 ± 0.064 | 0.339 ± 0.059 | 0.446 ± 0.023 |
| OT-10 | 0.365 ± 0.008 | 0.479 ± 0.038 | 0.607 ± 0.012 B | 0.410 ± 0.068 |
| OT-11 | 0.439 ± 0.065 | 0.406 ± 0.137 | 0.337 ± 0.076 | 0.345 ± 0.054 |
| OT-12 | 0.512 ± 0.017 AB | 0.329 ± 0.030 | 0.164 ± 0.086 E | 0.349 ± 0.159 |
| Fungi/Substrate | Crude Protein (%) | β-Glucan (mg/g) | ||
|---|---|---|---|---|
| Fermented Substrate | Untreated Substrate | Fermented Substrate | Untreated Substrate | |
| A. terreus/OT-01 | 23.3 ± 0.61 A | 10.9 ± 3.19 C | 490.99 ± 123.9 A | 372.62 ± 92.93 B |
| G. lucidum/OT-01 | 12.3 ± 0.89 C | 217.01 ± 26.11 D | ||
| P. pulmonarius/OT-07 | 9.27 ± 1.31 D | 8.31 ± 1.14 D | 542.9 ± 65.81 A | 286.16 ± 85.18 C |
| F. hepatica/OT-10 | 10.2 ± 2.37 C | 5.25 ± 0.71 E | 301.58 ± 86.9 C | 193.29 ± 43.7 D |
| P. lecomtei/OT-12 | 8.56 ± 0.97 D | 6.77 ± 2.52 D | 276.4 ± 21.43 C | 302.95 ± 26.15 C |
| A. terreus/CT-01 | 11.0 ± 0.86 C | 6.97 ± 0.38 D | 288.06 ± 73.9 C | 275.38 ± 42.67 C |
| G. lucidum/CT-07 | 11.2 ± 0.95 C | 7.31 ± 0.32 D | 122.22 ± 39.38 E | 472.61 ± 125.32 A |
| P. pulmonarius/CT-07 | 11.5 ± 0.33 C | 308.33 ± 81.46 C | ||
| P. lecomtei/CT-11 | 10.4 ± 1.74 C | 7.59 ± 1.38 D | 201.71 ± 58.29 D | 263.74 ± 34.44 C |
| P. lecomtei/CT-12 | 11.5 ± 1.10 C | 9.42 ± 2.20 D | 78.83 ± 1.5 E | 416.55 ± 37.8 B |
| P. lecomtei/ST-03 | 5.10 ± 0.59 E | 2.52 ± 1.67 E | 194.82 ± 17.24 D | 123.74 ± 10.47 E |
| P. lecomtei/ST-07 | 17.2 ± 1.82 B | 11.4 ± 3.40 C | 193.74 ± 50.8 D | 456.36 ± 32.56 A |
| P. lecomtei/ST-09 | 8.18 ± 0.20 D | 3.87 ± 0.23 E | 344.25 ± 23.13 C | 292.15 ± 9.611 C |
| A. terreus/ST-09 | 15.3 ± 0.46 B | 503.56 ± 8.6 A | ||
| P. lecomtei/ST-12 | 7.35 ± 0.83 D | 4.14 ± 0.30 E | 275.55 ± 31.22 C | 240.71 ± 6.78 C |
| Fermented Substrate | Lactone Form (mg/g) | β-Hydroxy Acid Form (mg/g) | Total (mg/g) |
|---|---|---|---|
| A. terreus in OT-01 | 0.03 | 0.25 | 0.29 ± 0.02 C |
| A. terreus in CT-01 | 0.15 | 0.54 | 0.69 ± 0.03 B |
| A. terreus in ST-09 | 0.17 | 0.93 | 1.10 ± 0.17 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conceição, A.A.; Mendes, T.D.; Mendonça, S.; Quirino, B.F.; Almeida, E.G.d.; Siqueira, F.G.d. Nutraceutical Enrichment of Animal Feed by Filamentous Fungi Fermentation. Fermentation 2022, 8, 402. https://doi.org/10.3390/fermentation8080402
Conceição AA, Mendes TD, Mendonça S, Quirino BF, Almeida EGd, Siqueira FGd. Nutraceutical Enrichment of Animal Feed by Filamentous Fungi Fermentation. Fermentation. 2022; 8(8):402. https://doi.org/10.3390/fermentation8080402
Chicago/Turabian StyleConceição, Aparecido Almeida, Thais Demarchi Mendes, Simone Mendonça, Betania Ferraz Quirino, Euziclei Gonzaga de Almeida, and Félix Gonçalves de Siqueira. 2022. "Nutraceutical Enrichment of Animal Feed by Filamentous Fungi Fermentation" Fermentation 8, no. 8: 402. https://doi.org/10.3390/fermentation8080402