The Impact of Must Nutrients and Yeast Strain on the Aromatic Quality of Wines for Cognac Distillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fermentations
2.2. Quantification of Sterols and Fatty Acids in Grape Solids
2.2.1. Dry Matter
2.2.2. Lipid Composition
2.3. Analytical Methods
2.4. Statistical Analysis
2.4.1. Experimental Central Composite Design
2.4.2. Analysis of Covariance (ANCOVA)
2.4.3. Analysis of Correlation Tree and PCA
3. Results and Discussion
3.1. Fermentation Kinetics
3.2. Compounds of Central Carbon Metabolism
3.3. Aromas
3.3.1. Fermentative Aromas
3.3.2. Terpenes
3.3.3. 1-octen-3-ol
3.3.4. Acetaldehyde
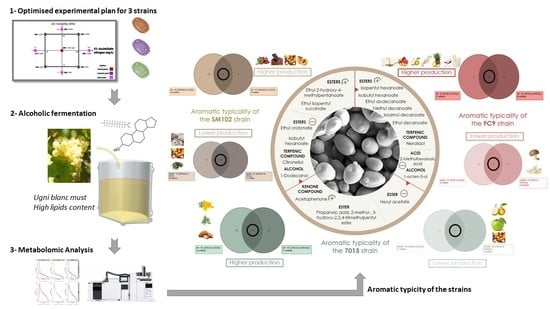
3.4. Aromatic Typicity of the Strains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of Key Odorant Compounds in Freshly Distilled Cognac Using GC-O, GC-MS, and Sensory Evaluation. J. Agric. Food Chem. 2004, 52, 7. [Google Scholar] [CrossRef] [PubMed]
- Lurton, L.; Snakkers, G.; Roulland, C.; Galy, B.; Versavaud, A. Influence of the fermentation yeast strain on the composition of wine spirits. J. Sci. Food Agric. 1995, 67, 485–491. [Google Scholar] [CrossRef]
- Vilanova, M.; Siebert, T.E.; Varela, C.; Pretorius, I.S.; Henschke, P.A. Effect of ammonium nitrogen supplementation of grape juice on wine volatiles and non-volatiles composition of the aromatic grape variety Albariño. Food Chem. 2012, 133, 124–131. [Google Scholar] [CrossRef]
- Casalta, E.; Cervi, M.F.; Salmon, J.M.; Sablayrolles, J.M. White wine fermentation: Interaction of assimilable nitrogen and grape solids: Interaction of assimilable nitrogen and grape solids on alcoholic fermentation under oenological conditions. Aust. J. Grape Wine Res. 2013, 19, 47–52. [Google Scholar] [CrossRef]
- Beltran, G.; Esteve-Zarzoso, B.; Rozès, N.; Mas, A.; Guillamón, J.M. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 2005, 53, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Guittin, C.; Maçna, F.; Sanchez, I.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. Impact of high lipid contents on the production of fermentative aromas during white wine fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 6435–6449. [Google Scholar] [CrossRef]
- Jiménez-Martí, E.; Aranda, A.; Mendes-Ferreira, A.; Mendes-Faia, A.; lí del Olmo, M. The nature of the nitrogen source added to nitrogen depleted vinifications conducted by a Saccharomyces cerevisiae strain in synthetic must affects gene expression and the levels of several volatile compounds. Antonie Leeuwenhoek 2007, 92, 61–75. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.-M.; Dequin, S.; Mouret, J.-R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef]
- Seguinot, P.; Rollero, S.; Sanchez, I.; Sablayrolles, J.-M.; Ortiz-Julien, A.; Camarasa, C.; Mouret, J.-R. Impact of the timing and the nature of nitrogen additions on the production kinetics of fermentative aromas by Saccharomyces cerevisiae during winemaking fermentation in synthetic media. Food Microbiol. 2018, 76, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Leigh Francis, I.; Henschke, P.A. Comparison of inorganic and organic nitrogen supplementation of grape juice—Effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Torrea, D.; Henschke, P.A. Ammonium supplementation of grape juice-effect on the aroma profile of a Chardonnay wine. TechRev 2004, 150, 59–63. [Google Scholar]
- Mouret, J.R.; Camarasa, C.; Angenieux, M.; Aguera, E.; Perez, M.; Farines, V.; Sablayrolles, J.M. Kinetic analysis and gas–liquid balances of the production of fermentative aromas during winemaking fermentations: Effect of assimilable nitrogen and temperature. Food Res. Int. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007, 77, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.C.; Wolff, S.R.; Bisson, L.F.; Ebeler, S.E. Yeast Strain and Nitrogen Supplementation: Dynamics of Volatile Ester Production in Chardonnay Juice Fermentations. Am. J. Enol. Vitic. 2007, 58, 470–483. [Google Scholar]
- Andreasen, A.A.; Stier, T.J.B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell. Physiol. 1953, 41, 23–36. [Google Scholar] [CrossRef]
- Casalta, E.; Salmon, J.-M.; Picou, C.; Sablayrolles, J.-M. Grape Solids: Lipid Composition and Role during Alcoholic Fermentation under Enological Conditions. Am. J. Enol. Vitic. 2019, 70, 147–154. [Google Scholar] [CrossRef]
- Rollero, S.; Mouret, J.-R.; Sanchez, I.; Camarasa, C.; Ortiz-Julien, A.; Sablayrolles, J.-M.; Dequin, S. Key role of lipid management in nitrogen and aroma metabolism in an evolved wine yeast strain. Microb. Cell Factories 2016, 15, 32. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Yunoki, K.; Yasui, Y.; Hirose, S.; Ohnishi, M. Fatty acids in must prepared from 11 grapes grown in Japan: Comparison with wine and effect on fatty acid ethyl ester formation. Lipids 2005, 40, 361–367. [Google Scholar] [CrossRef]
- Sablayrolles, J.M.; Barre, P.; Grenier, P. Design of a laboratory automatic system for studying alcoholic fermentations in anisothermal enological conditions. Biotechnol. Tech. 1987, 1, 181–184. [Google Scholar] [CrossRef]
- Crépin, L.; Nidelet, T.; Sanchez, I.; Dequin, S.; Camarasa, C. Sequential Use of Nitrogen Compounds by Saccharomyces cerevisiae during Wine Fermentation: A Model Based on Kinetic and Regulation Characteristics of Nitrogen Permeases. Appl. Environ. Microbiol. 2012, 78, 8102–8111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenth, R.V. Response-Surface Methods in R, Using rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B 1951, 13, 1–38. [Google Scholar] [CrossRef]
- Manginot, C.; Sablayrolles, J.M.; Roustan, J.L.; Barre, P. Use of constant rate alcoholic fermentations to compare the effectiveness of different nitrogen sources added during the stationnary phase. Enz. Microbiol. Tech. 1997, 20, 373–380. [Google Scholar] [CrossRef]
- Blateyron, L.; Sablayrolles, J. Stuck and Slow Fermentations in Enology: Statistical Study of Causes and Effectiveness of Combined Additions of Oxygen and Diammonium Phosphate. J. Biosci. Bioeng. 2001, 91, 184–189. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Description of Alcoholic Fermentation Kinetics: Its Variability and Significance. Am. J. Enol. Vitic. 1990, 41, 319–324. [Google Scholar]
- Ochando, T.; Mouret, J.-R.; Humbert-Goffard, A.; Sablayrolles, J.-M.; Farines, V. Impact of initial lipid content and oxygen supply on alcoholic fermentation in champagne-like musts. Food Res. Int. 2017, 98, 87–94. [Google Scholar] [CrossRef]
- Deroite, A.; Legras, J.-L.; Rigou, P.; Ortiz-Julien, A.; Dequin, S. Lipids modulate acetic acid and thiol final concentrations in wine during fermentation by Saccharomyces cerevisiae × Saccharomyces kudriavzevii hybrids. AMB Expr. 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Luparia, V.; Soubeyrand, V.; Berges, T.; Julien, A.; Salmon, J.-M. Assimilation of grape phytosterols by Saccharomyces cerevisiae and their impact on enological fermentations. Appl. Microbiol. Biotechnol. 2004, 65, 25–32. [Google Scholar] [CrossRef]
- Rollero, S. Impact des paramètres environnementaux sur la synthèse des arômes fermentaires par Saccharomyces cerevisiae en fermentation oenologique. Ph.D. Thesis, Montpellier SupAgro, Montpellier, France, 2015. [Google Scholar]
- Albers, E.; Larsson, C.; Lidén, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remize, F.; Sablayrolles, J.M.; Dequin, S. Re-assessment of the influence of yeast strain and environmental factors on glycerol production in wine. J. Appl. Microbiol. 2000, 88, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Nykanen, I. Aroma of Beer, Wine and Distilled Alcoholic Beverages; Kluwer Academic Publishers: Berlin, Germany, 1983. [Google Scholar]
- Verstrepen, K.J.; Laere, S.D.M.V.; Vanderhaegen, B.M.P.; Derdelinckx, G.; Dufour, J.-P.; Pretorius, I.S.; Winderickx, J.; Thevelein, J.M.; Delvaux, F.R. Expression Levels of the Yeast Alcohol Acetyltransferase Genes ATF1, Lg-ATF1, and ATF2 Control the Formation of a Broad Range of Volatile Esters. Appl. Environ. Microbiol. 2003, 69, 5228–5237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, T.; Kobayashi, O.; Yoshimoto, H.; Furukawa, S.; Tamai, Y. Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces cerevisiae alcohol acetyltransferase gene. Appl. Environ. Microbiol. 1997, 63, 910–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, D.; Yoshimoto, H.; Sone, H.; Harashima, S.; Tamai, Y. Transcriptional co-regulation of Saccharomyces cerevisiae alcohol acetyltransferase gene, ATF1 and Δ-9 fatty acid desaturase gene, OLE1 by unsaturated fatty acids. Yeast 1998, 14, 711–721. [Google Scholar] [CrossRef]
- Torrea, D. Production of volatile compounds in the fermentation of chardonnay musts inoculated with two strains of Saccharomyces cerevisiae with different nitrogen demands. Food Control 2003, 14, 565–571. [Google Scholar] [CrossRef]
- Ugliano, M.; Travis, B.; Francis, I.L.; Henschke, P.A. Volatile Composition and Sensory Properties of Shiraz Wines as Affected by Nitrogen Supplementation and Yeast Species: Rationalizing Nitrogen Modulation of Wine Aroma. J. Agric. Food Chem. 2010, 58, 12417–12425. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Torrea, D.; Schmidt, S.A.; Ancin-Azpilicueta, C.; Henschke, P.A. Effect of oxygen and lipid supplementation on the volatile composition of chemically defined medium and Chardonnay wine fermented with Saccharomyces cerevisiae. Food Chem. 2012, 135, 2863–2871. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances: Terpenoids: Role in wine flavour. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Dubourdieu, D.; Glories, Y.; Maujean, A. Traitéd’oenologie: Chimie Du Vin, Stabilisation et Traitements; Dunod: Malakoff, France, 2017. [Google Scholar]
- Malfondet, N.; Gourrat, K.; Brunerie, P.; Le Quéré, J.-L. Aroma characterization of freshly-distilled French brandies; their specificity and variability within a limited geographic area: Aroma characterization of freshly-distilled French brandies. Flavour Fragr. J. 2016, 31, 361–376. [Google Scholar] [CrossRef]
- Schreier, P.; Drawert, F.; Winkler, F. Composition of neutral volatile constituents in grape brandies. J. Agric. Food Chem. 1979, 27, 365–372. [Google Scholar] [CrossRef]
- Thibaud, F.; Courregelongue, M.; Darriet, P. Contribution of Volatile Odorous Terpenoid Compounds to Aged Cognac Spirits Aroma in a Context of Multicomponent Odor Mixtures. J. Agric. Food Chem. 2020, 68, 13310–13318. [Google Scholar] [CrossRef] [PubMed]
- Eliseev, M.; Gribkova, I.; Kosareva, O.; Alexeyeva, O. Effect of organic compounds on cognac sensory profile. Foods Raw Mater. 2021, 244–253. [Google Scholar] [CrossRef]
- Wilson, B.; Strauss, C.R.; Williams, J. The Distribution of Free and Glycosidically-Bound Monoterpenes among Skin, Juice, and Pulp Fractions of Some White Grape Varieties. Am. J. Enol. Vitic. 1986, 37, 107–111. [Google Scholar]
- Charoenchai, C.; Fleet, G.H.; Henschke, P.A.; Todd, B.E.N.T. Screening of non-Saccharomyces wine yeasts for the presence of extracellular hydrolytic enzymes. Aust. J. Grape Wine Res. 1997, 3, 2–8. [Google Scholar] [CrossRef]
- Strauss, M.L.A.; Jolly, N.P.; Lambrechts, M.G.; van Rensburg, P. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J. Appl. Microbiol. 2001, 91, 182–190. [Google Scholar] [CrossRef]
- Awad, P.; Athès, V.; Decloux, M.E.; Ferrari, G.; Snakkers, G.; Raguenaud, P.; Giampaoli, P. Evolution of Volatile Compounds during the Distillation of Cognac Spirit. J. Agric. Food Chem. 2017, 65, 7736–7748. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynen, F. The pathway from activated acetic acid to the terpenes and fatty acids. Proc. R. Caroline Inst. 1964, 103–138. [Google Scholar]
- Gamero, A.; Manzanares, P.; Querol, A.; Belloch, C. Monoterpene alcohols release and bioconversion by Saccharomyces species and hybrids. Int. J. Food Microbiol. 2011, 145, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Steyer, D. Genetic analysis of geraniol metabolism during fermentation. Food Microbiol. 2013, 7, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Vaudano, E.; Moruno, E.G.; Stefano, R. Modulation of Geraniol Metabolism during Alcohol Fermentation. J. Inst. Brew. 2004, 110, 213–219. [Google Scholar] [CrossRef]
- Denat, M.; Pérez, D.; Heras, J.M.; Querol, A.; Ferreira, V. The effects of Saccharomyces cerevisiae strains carrying alcoholic fermentation on the fermentative and varietal aroma profiles of young and aged Tempranillo wines. Food Chem. X 2021, 9, 100116. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Güntert, M.; Engel, K.-H. (Eds.) Aroma Active Compounds in Foods: Chemistry and Sensory Properties; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; Volume 794, ISBN 978-0-8412-3694-3. [Google Scholar]
- Pallotta, U.; Castellari, M.; Piva, A.; Baumes, R.; Bayonove, C. Effects of Botrytis cinerea on must composition of three italian grape varieties. Wein-Wissenschaft 1998, 53, 32–36. [Google Scholar]
- La Guerche, S.; Dauphin, B.; Pons, M.; Blancard, D.; Darriet, P. Characterization of Some Mushroom and Earthy Off-Odors Microbially Induced by the Development of Rot on Grapes. J. Agric. Food Chem. 2006, 54, 9193–9200. [Google Scholar] [CrossRef]
- Wurzenberger, M.; Grosch, W. The formation of 1-octen-3-ol from the 10-hydroperoxide isomer of linoleic acid by a hydroperoxide lyase in mushrooms (Psalliota bispora). Biochim. Biophys. Acta Lipids Lipid Metab. 1984, 794, 25–30. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Pilone, G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT Food Sci. Technol. 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Wucherpfenning, K.; Semmler, G. Formation of acetaldehyde during fermentation in relation to pH-value and to individual vitamins. Z. Lebensm. Unters. Forsch. 1972, 148, 138–145. [Google Scholar]
- Bosso, A.; Guaita, M. Study of some factors involved in ethanal production during alcoholic fermentation. Eur. Food Res. Technol. 2008, 227, 911–917. [Google Scholar] [CrossRef]




| Name | Formula | mg/g of Dry Matter | mg/L of Grape Solids |
|---|---|---|---|
| Lauric | C12:0 | 0.03 | 0.82 |
| Myristic | C14:0 | 0.12 | 3.24 |
| Pentadecylic | C15:0 | 0.06 | 1.46 |
| Palmitic | C16:0 | 8.29 | 215.07 |
| Palmitoleic | C16:1 | 0.14 | 3.51 |
| Margaric | C17:0 | 0.08 | 2.04 |
| Stearic | C18:0 | 0.93 | 24.23 |
| Oleic | C18:1 | 2.95 | 76.49 |
| Linoleic | C18:2 | 17.69 | 458.83 |
| Linolenic | C18:3 | 4.43 | 114.84 |
| Arachidic | C20:0 | 0.36 | 9.24 |
| Gondoic | C20:1 | 0.07 | 1.94 |
| Heneicosylic | C21:0 | 0.12 | 3.12 |
| Behenic | C22:0 | 1.00 | 25.85 |
| Tricosylic | C23:0 | 0.13 | 3.34 |
| Lignoceric | C24:0 | 0.32 | 8.30 |
| Total | 37.28 | 897.57 |
| Name | mg/g of Dry Matter | mg/L of Grape Solids |
|---|---|---|
| Campesterol | 0.51 | 13.24 |
| Stigmasterol | 0.39 | 10.12 |
| β-sitosterol | 7.01 | 181.80 |
| Sitostanol | 0.24 | 6.34 |
| Unidentified sterols | 0.28 | 7.1 |
| Total | 8.43 | 218.60 |
| Independent Variable | ||||
|---|---|---|---|---|
| Experiments | Assimilable Nitrogen (mgN/L) | Turbidity (NTU) | ||
| Coded Level | Uncoded Level | Coded Level | Uncoded Level | |
| 1 | −1 | 140 | −1 | 820 |
| 2 | +1 | 260 | −1 | 820 |
| 3 | −1 | 140 | +1 | 2380 |
| 4 | +1 | 260 | +1 | 2380 |
| 5 a | 0 | 200 | 0 | 1600 |
| 6 a | 0 | 200 | 0 | 1600 |
| 7 a | 0 | 200 | 0 | 1600 |
| 8 a | 0 | 200 | 0 | 1600 |
| 9 | −1.41 | 115 | 0 | 1600 |
| 10 | +1.41 | 285 | 0 | 1600 |
| 11 | 0 | 200 | −1.41 | 500 |
| 12 | 0 | 200 | +1.41 | 2700 |
| Kinetic Parameters | MCC Compounds | ||||||
|---|---|---|---|---|---|---|---|
| Strain | Effect | Variable | Vmax | Time of Fermentation | Glycerol | Succinate | α-Ketoglutarate |
| FC9® | Simple effects | Nass | ↗ | ↘ | ↗ | ↘ | ↘ |
| NTU | ↗ | ↗ | ↗ | ↗ | |||
| Interaction effects | Nass:NTU | ↗ | ↗ | ||||
| Quadratic effects | Nass | ↘ | ↗ | ||||
| NTU | |||||||
| 7013® | Simple effects | Nass | ↗ | ↘ | ↗ | ↘ | |
| NTU | ↗ | ↗ | |||||
| Interaction effects | Nass:NTU | ||||||
| Quadratic effects | Nass | ↘ | ↗ | ||||
| NTU | |||||||
| SM102® | Simple effects | Nass | ↗ | ↗ | ↘ | ↘ | |
| NTU | ↗ | ↗ | ↗ | ↗ | |||
| Interaction effects | Nass:NTU | ↗ | ↗ | ↗ | |||
| Quadratic effects | Nass | ↘ | ↘ | ↘ | |||
| NTU | ↘ | ↘ | ↘ | ||||
| ANCOVA (p-value): « strain effect » | +++ | +++ | +++ | +++ | +++ | ||
| Strain | Effect | Variable | Sum of Higher Alcohols | Sum of Acetate Esters |
|---|---|---|---|---|
| FC9® | Simple effects | Nass | ↘ | ↗ |
| NTU | ↗ | ↘ | ||
| Interaction effects | Nass:NTU | ↗ | ||
| Quadratic effects | Nass | |||
| NTU | ||||
| 7013® | Simple effects | Nass | ↘ | ↗ |
| NTU | ↗ | ↘ | ||
| Interaction effects | Nass:NTU | |||
| Quadratic effects | Nass | ↘ | ||
| NTU | ↘ | ↗ | ||
| SM102® | Simple effects | Nass | ↘ | ↗ |
| NTU | ↗ | ↘ | ||
| Interaction effects | Nass:NTU | |||
| Quadratic effects | Nass | |||
| NTU | ↗ | |||
| ANCOVA (p-value): « strain effect » | +++ | +++ | ||
| Strain | Effect | Variable | Ethyl Isobutanoate | Ethyl Butanoate | Ethyl Hexanoate | Ethyl Octanoate | Ethyl Decanoate |
|---|---|---|---|---|---|---|---|
| FC9® | S.E | Nass | ↘ | ||||
| NTU | ↘ | ↘ | |||||
| I.E | Nass:NTU | ||||||
| Q.E | Nass | ↗ | |||||
| NTU | ↗ | ↗ | |||||
| 7013® | S.E | Nass | ↘ | ↗ | ↗ | ↗ | |
| NTU | ↗ | ↘ | ↘ | ↘ | ↘ | ||
| I.E | Nass:NTU | ↘ | |||||
| Q.E | Nass | ↗ | |||||
| NTU | ↗ | ↗ | ↗ | ||||
| SM102® | S.E | Nass | ↗ | ||||
| NTU | ↗ | ↘ | ↘ | ↘ | |||
| I.E | Nass:NTU | ↘ | |||||
| Q.E | Nass | ||||||
| NTU | ↗ | ↗ | |||||
| ANCOVA (p-value): « strain effect » | +++ | +++ | +++ | +++ | +++ | ||
| Strain | Effect | Variable | Propionic Acid | Pentanoic Acid | Octanoic Acid | Decanoic Acid | Hexanoic Acid |
|---|---|---|---|---|---|---|---|
| FC9® | S.E | Nass | ↘ | ||||
| NTU | ↘ | ↘ | ↘ | ||||
| I.E | Nass:NTU | ||||||
| Q.E | Nass | ↗ | |||||
| NTU | ↗ | ||||||
| 7013® | S.E | Nass | ↘ | ↗ | ↗ | ↗ | |
| NTU | ↘ | ↘ | ↘ | ||||
| I.E | Nass:NTU | ↘ | |||||
| Q.E | Nass | ↗ | ↘ | ↘ | |||
| NTU | ↗ | ↗ | ↗ | ||||
| SM102® | S.E | Nass | ↘ | ↘ | ↗ | ↗ | ↗ |
| NTU | ↘ | ||||||
| I.E | Nass:NTU | ↘ | |||||
| Q.E | Nass | ↗ | ↗ | ↘ | ↗ | ||
| NTU | ↗ | ↗ | |||||
| ANCOVA (p-value): « strain effect » | +++ | +++ | +++ | +++ | +++ | ||
| Terpenes | Ketone | ||||||
|---|---|---|---|---|---|---|---|
| Strain | Effect | Variable | Trans-Nerolidol | Citronellol | α-Terpineol | Linalool | Acetophenone |
| FC9® | Simple effects | Nass | ↘ | ||||
| Interaction effects | Nass:NTU | ↗ | |||||
| Quadratic effects | Nass | ↗ | |||||
| 7013® | Simple effects | Nass | ↘ | ↘ | ↘ | ↗ | |
| NTU | ↗ | ↘ | |||||
| Quadratic effects | Nass | ↘ | ↘ | ||||
| NTU | ↘ | ||||||
| SM102® | Simple effects | Nass | ↘ | ↘ | ↘ | ↗ | |
| NTU | ↗ | ↗ | ↘ | ||||
| Interaction effects | Nass:NTU | ↘ | |||||
| Quadratic effects | Nass | ↘ | |||||
| ANCOVA (p-value): « strain effect » | +++ | +++ | +++ | +++ | +++ | ||
| Strain | Effect | Variable | 1-Octen-3-ol | 2-Nonanol |
|---|---|---|---|---|
| FC9® | Simple effects | Nass | ||
| NTU | ||||
| Interaction effects | Nass:NTU | ↗ | ||
| Quadratic effects | Nass | ↗ | ||
| NTU | ||||
| 7013® | Simple effects | Nass | ||
| NTU | ↗ | ↗ | ||
| Interaction effects | Nass:NTU | ↘ | ||
| Quadratic effects | Nass | ↗ | ||
| NTU | ↗ | |||
| SM102® | Simple effects | Nass | ||
| NTU | ↗ | |||
| Interaction effects | Nass:NTU | ↘ | ||
| Quadratic effects | Nass | |||
| NTU | ↘ | |||
| ANCOVA (p-value): « strain effect » | / | +++ | ||
| Strain | Effect | Variable | Acetaldehyde | Benzaldehyde |
|---|---|---|---|---|
| FC9® | Simple effects | Nass | ↗ | |
| NTU | ||||
| Interaction effects | Nass:NTU | |||
| Quadratic effects | Nass | |||
| NTU | ||||
| 7013® | Simple effects | Nass | ↗ | ↘ |
| NTU | ↘ | ↗ | ||
| Interaction effects | Nass:NTU | ↘ | ||
| Quadratic effects | Nass | ↘ | ||
| NTU | ||||
| SM102® | Simple effects | Nass | ↗ | ↘ |
| NTU | ↘ | |||
| Interaction effects | Nass:NTU | ↘ | ||
| Quadratic effects | Nass | ↗ | ||
| NTU | ||||
| ANCOVA (p-value): « strain effect » | +++ | / | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guittin, C.; Maçna, F.; Sanchez, I.; Barreau, A.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. The Impact of Must Nutrients and Yeast Strain on the Aromatic Quality of Wines for Cognac Distillation. Fermentation 2022, 8, 51. https://doi.org/10.3390/fermentation8020051
Guittin C, Maçna F, Sanchez I, Barreau A, Poitou X, Sablayrolles J-M, Mouret J-R, Farines V. The Impact of Must Nutrients and Yeast Strain on the Aromatic Quality of Wines for Cognac Distillation. Fermentation. 2022; 8(2):51. https://doi.org/10.3390/fermentation8020051
Chicago/Turabian StyleGuittin, Charlie, Faïza Maçna, Isabelle Sanchez, Adeline Barreau, Xavier Poitou, Jean-Marie Sablayrolles, Jean-Roch Mouret, and Vincent Farines. 2022. "The Impact of Must Nutrients and Yeast Strain on the Aromatic Quality of Wines for Cognac Distillation" Fermentation 8, no. 2: 51. https://doi.org/10.3390/fermentation8020051