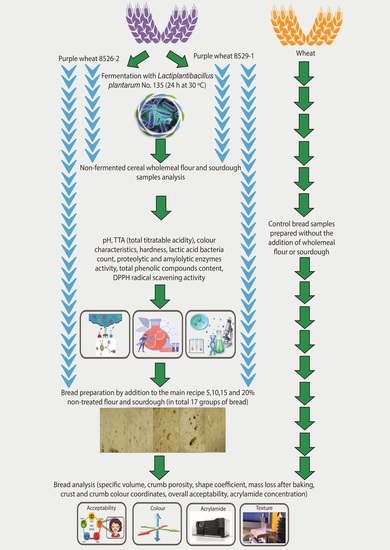
3.1. Parameters of the Non-Treated Purple Wheat Grain Wholemeal and Sourdough Samples
Acidity characteristics (pH and TTA), LAB count, colour coordinates, and hardness of non-treated and fermented purple wheat grain wholemeal samples are shown in
Table 2, as well as enzyme activities in
Table 3. Significant differences in pH were not found between non-treated and fermented wheat cereal varieties, and mean pH values were 5.59 in the non-treated group and 3.75 in the fermented samples. Although TTA did not differ significantly between fermented sample groups, the fermented 8529-1 variety showed 18.85% higher TTA than the fermented 8526-2 variety. Fermentation, wheat variety, and their interaction had significant effects on TTA (
Table 4); however, significant correlations between pH and TTA were not found. These tendencies can be explained by the different wheat varieties’ different buffering effects. It has been reported that the outer parts of the cereal grains contain compounds that act as buffering agents [
43]. Higher quantities of the compounds with buffering characteristics in the fermentable substrate lead to organic acid neutralization, and in substrates with less buffering agents a low pH will be reached with lower organic acid production.
No significant different in LAB count were found between different varieties in the non-treated and fermented cereal wholemeal sample groups. Average LAB count in non-fermented samples was 4.45 log
10 CFU/g, and in fermented ones, 8.38 log
10 CFU/g. Usually, when mean LAB count in sourdough is 10
8 CFU/g, sourdough is characterized as stable in terms of LAB domination and physical-chemical properties (e.g., acidification rate, organic acid production, etc.) [
44]. However, the changes over time should be controlled if this sourdough will be used further as a mother sourdough.
For colour characteristics, higher L* (lightness) coordinates were found in fermented samples (both 8526-2 and 8526-1) than in non-treated samples (by 31.13 and 19.61%, respectively). Non-treated and fermented samples of 8526-2 had higher a* (redness) coordinates than 8526-1 by 19.37 and 16.67%, respectively. B* (yellowness) were higher in 8526-2 than in 8526-1 samples by 10.71 and 11.39% for non-treated and fermented samples, respectively. A moderate significant positive correlation was found between samples’ L* and LAB count (r = 0.611,
p = 0.035). Although there was no correlation between LAB count and TTA, there was a significant positive correlation between L* coordinate and TTA (r = 0.766,
p = 0.004). These findings can be explained by the low stability of anthocyanins. By increasing TTA, anthocyanin content may have been reduced, leading to a higher L* coordinate of the fermentable substrate. Overall, the stability of anthocyanins is affected by temperature, pH, light, and composition of the fermentable substrate [
45]. Moreover, this study showed that the wheat cereal varieties used in this experiment, had different buffering capacities, and that increasing LAB counts in substrate were related to a higher L* coordinate. However, no correlation between LAB count and TTA was found.
In both wheat varieties, fermentation reduced sample texture hardness, but no correlations were found between texture hardness and any of the analysed parameters (acidity, LAB count, amylolytic and proteolytic enzymes activity). The decrease in hardness of the wholemeal flour during fermentation can be explained by complex interactions, including competition for water by the water-soluble and water-insoluble fiber constituents, starch degradation, etc. The fiber, or non-starch polysaccharide, fraction, of whole wheat is composed primarily of arabinoxylans [
46,
47]. During fermentation, these compounds are broken down into lower-molecular-weight substances, and these changes can lead to a lower-hardness fermentable substrate.
Significant differences in proteolytic and amylolytic enzyme activities were not found between the different wheat varieties and treatments (
Table 3). Proteolytic enzyme activity was, on average, 144.3 PU, and average amylolytic enzyme activity was 156.3 AU. Wheat flour is a raw material, which, in addition to the main constituents, also includes a variety of enzymes. However, microorganisms are considered the main source of enzymes because their reproduction rate is high and they excrete bioactive compounds, including various enzymes, into the fermentable substrate [
48]. In wheat flour, α-amylase activity is low, and β-amylases are abundant but have little or low activity [
48]. The influence of cereal fermentation is associated with organic acid synthesis, activation of the flour endogenous enzymes, and microbial secondary metabolic activity [
49,
50,
51]. In addition, LAB possess a variety of enzymatic activities [
52,
53,
54], although this characteristic is strain-specific [
55,
56]. This study found no significant differences between the amylolytic and proteolytic enzyme activities of fermented and non-treated purple wheat wholemeal samples.
The lowest TPC content occurred in non-fermented 8526-2 samples (52.9 mg/100 g d.m.); however, after fermentation TCP was increased, on average, by 52.3% (
Table 3). Significant differences in TPC content between non-treated and fermented 8526-2 samples were not found, and TPC content was, on average, 110.8 mg/100 g d.m.
In all cases fermentation increased antioxidant DPPH-radical-scavenging activity of the samples—in 8526-2 samples, on average, by 9.32 times, and in 8526-1 samples, on average, by 1.95 times (
Table 3). A very strong positive correlation between TPC content and DPPH-radical-scavenging activity was found (r = 0.816,
p = 0.001).
It has been reported that cereals and cereal-based products contain significant levels of antioxidants [
57,
58], and fermentation of cereals can enhance these properties as well [
59]. However, although fermentation has a positive influence on TPC and antioxidative activity of cereals, the degree of influence depends on the microorganism used to treat the cereal grain [
60]. Ðordević et al. [
59] reported no correlation between TPC content and DPPH-radical-scavenging activity in cereals. However, this study was performed with traditional wheat (
Triticum durum), and, according to Brand-Williams, Cuvelier, and Berset [
61], ferulic acid, the main phenolic acid in traditional wheat grains, showed a weak antiradical effect in experiments with the DPPH radical, which may explain the discrepancies. However, in our study the tested coloured wheat antioxidant properties can be associated with other compounds (pigments, e.g., anthocyanins), and this can explain the different results obtained and the correlation between the coloured wheat grain wholemeal TPC content and DPPH. Finally, the use of fermentation as a separate process can enhance the levels of antioxidants in coloured wheat grain wholemeal and can be used to improve its functional properties.
3.2. Bread Quality Characteristics
Bread specific volume, porosity, shape coefficient, mass loss after baking, and bread crumb images are shown in
Table 5. The highest bread specific volume occurred in the control bread samples, 8526-2 N-T-5% and 8526-2 F-15% (on average, 2.92 cm
3/g). Bread with 5% of the non-treated 8526-2 showed a 6.25% higher specific volume than 8526-2 F-5% samples. However, significant differences between bread samples prepared with 10% of the non-treated and fermented 8526-2 were not found. Fermentation led to a 24.0% higher specific volume of the bread enriched with 15% of 8526-2 sourdough compared to bread prepared with non-treated 8526-2 wholemeal. However, opposite tendencies were found in the bread enriched with 20% of 8526-2 sourdough: both non-treated and fermented 8526-2 addition at 20% led to significant bread specific volume reduction. Bread prepared with both non-treated and fermented 8529-1 wheat wholemeal had higher specific volume at 5, 10, and 15% addition levels, but lower at 20%.However, the analysed factors and their interactions did not significantly affect bread specific volume (
Table 6).
Samples 8526-2 N-T-15%, 8526-2 N-T-20%, 8529-1 N-T-5%, 8529-1 N-T-10%, 8529-1 N-T-15%, 8529-1 N-T-20%, 8529-1 F-5%, and 8529-1 F-10% showed, on average, 8.76% lower porosity than samples 8526-2 N-T-5%, 8526-2 N-T-10%, 8526-2 F-5%, 8526-2 F-10%, 8526-2 F-15%, 8526-2 F-20%, 8529-1 F-15%, and 8529-1 F-20%. No analysed factors had significant effects on bread porosity (
Table 6).
By increasing the non-treated 8526-2 wheat wholemeal quantity in the main bread formula, the shape coefficient of the bread was reduced; however, the addition of 15% 8526-2 sourdough increased the bread shape coefficient compared to samples prepared with 5 and 10%. Samples with non-treated 8529-1 wholemeal showed higher shape coefficients than samples prepared with the same quantities of 8529-1 wholemeal sourdough. However, the analysed factors and their interactions did not significantly affect the bread shape coefficient (
Table 6).
The highest mass lost after baking was showed by control samples and samples prepared with 15% 8529 wholemeal sourdough. Fermentation, as well as the interaction of non-fermented/fermented wheat wholemeal and wheat variety, significantly affected bread mass after baking (
p = 0.032 and
p = 0.028, respectively) (
Table 6).
Bread production is a very complex process and the measurement of rheological parameters assists in controlling the behaviour of dough and the quality of the final bread [
62]. The inclusion of various types of dietary fibers, e.g., wholemeal flour, in bread production significantly influences both processing and quality of bread [
62]. Dietary fibers can interfere with protein association, weaken the dough, and affect gelling and pasting [
63]. There is a wide range of studies on the physical parameters of bread with dietary fibers. In most cases, negative effects of dietary fibers on bread volume and moisture loss were observed and attributed to gluten dilution and lower gas retention [
62,
64,
65,
66]. A similar tendency was also found in our study. Moreover, the degree of substrate breakdown, acidification properties, and metabolism of LAB affect the quality of bread made with sourdough. The drop in pH due to LAB activity during sourdough fermentation causes swelling of gluten and arabinoxylans as well as hydrolysis of starch [
67]. This also accelerates the activity of not only LAB proteolytic enzymes, which induce gluten proteolysis, but also endogenous cereal enzymes [
50]. This is significant for bread volume, gas retention, and dough rheology. Reduction of gluten viscosity during prolonged fermentation as well as increased degree of softening and lower resistance to extension were observed in fermented doughs [
67]. However, other studies reported improved bread texture of wheat bread with sourdough [
67]. Other compounds produced by LAB during fermentation, such as exopolysaccharides, glucose, mannitol, and acetate, could be related to increased loaf volume, water absorption of the dough, and delayed bread staling [
10]. According to Sun et al.
L. plantarum is usually chosen due to its ability to significantly elicit both decline and expansion in the hardness, cohesiveness, and viscoelasticity of whole-wheat bread [
68]. In our study, the pH was lower in bread with both wheat wholemeal varieties fermented with
L. plantarum, but proteolytic and amylolytic enzyme activities were similar in both non-treated and fermented samples. This could partly contribute to the observed changes in bread quality attributes.
3.3. The Changes of Bread Texture Hardness during the Storage
After 12 h of storage, samples prepared with 5% non-treated 8526-2 wholemeal showed lower hardnesscompared to control samples (
Figure 3).
Similar tendencies were found in the samples prepared with 5% non-treated 8526-1 wholemeal: their hardness was the same as that of controls (0.5 mJ). However, by increasing non-treated and fermented coloured wheat wholemeal content in the main bread formula, bread hardness was increased.
After 48 h of storage, most of the samples showed higher hardness in comparison with control breads, except for 8526-2 F-5%, whose hardness was, on average, 12.5% lower, and 8526-2 F-10% and 8529-1 N-T-5%, whose hardness did not differ from the control value of 0.8 mJ.
After 72 h of storage, samples 8526-2 N-T-5%, 8526-2 F-5%, 8526-2 F-10%, 8529-1 N-T-5%, and 8529-1 F-5% showed lower hardness than that of control samples. However, the analysed factors and their interactions did not significantly affect bread hardness during storage (
Table 7).
Bread staling is caused by the retrogradation of amylose and amylopectin [
69]. Dietary fibers in wholemeal could merge these compounds in order to slow down the staling process, but the effect mainly depends on the type, content, and particle size of dietary fibers [
70,
71]. The addition of DF induces denser and firmer texture in bread [
72]. A higher content of insoluble fiber in bread could increase bread firmness during storage [
73] and this tendency was also observed in our study. A number of studies reported that dietary fibers improved the shelf-life of bread, while the application of sourdough when baking with wheat has been found to have mixed effects on the shelf-life of wheat bread [
74]. The positive effect of purple wheat sourdough on the delay of wheat bread staling could be explained by the production of certain metabolites and the enzymatic activity of LAB. Exopolysaccharides act as hydrocolloids and result in greater water retention and softer crumb structure, while organic acids enhance amylase and protease activities, thus decreasing the staling rate [
75].
3.4. Bread Overall Acceptability, Crust and Crumb Colour Coordinates and Acrylamide Concentration
Bread overall acceptability is shown in
Figure 4, crust and crumb colour coordinates are given in
Table 8 and acrylamide concentration is shown in
Figure 5. In most cases, addition of non-treated and fermented coloured wheat cereal grain wholemeal at 15% to the main bread formula increased overall bread acceptability, in comparison with the control group and groups prepared with 5, 10, and 20% of non-treated and fermented coloured wheat cereal grain wholemeal (
Figure 4). Significant differences in overall acceptability were not found between non-fermented and fermented groups with the same quantity of wheat wholemeal added, and the analysed factors and their interactions did not significantly affect overall bread acceptability (
Table 9).
Similar to our results, other studies also reported that additions of such dietary fibers sources as wheat bran to bread formula had a positive effect on sensory properties of baked products [
62,
76,
77,
78]. Sourdough bread contains a greater amount of volatile compounds and that could result in higher scores in sensory tests [
79]. According to the results of our study, by adding a higher quantity of non-treated or fermented purple wheat sourdough to bread, its acceptability was enhanced. The use of sourdough in bread making enhances texture and flavor attributes, leading to better consumer acceptance [
80]. Acidification, protein hydrolysis, and release of phenolic compounds during sourdough fermentation contribute to bread flavor formation [
68]. Furthermore, fermentation with LAB can reduce bitterness in breads prepared with wholemeal flour and increase fruitiness taste [
81]. Mantzourani et al. reported that sourdough fermented with
Lacticaseibacillus paracasei K5 enhanced bread sensory properties and acceptability to consumers [
82].
Colour characteristics of the bread crust and crumb are shown in
Table 8. In all the cases, by including coloured wheat wholemeal to the main bread formula, bread crust L*, a*, and b* coordinates were increased. Significant differences between samples in the crumb L* coordinates were not found; however, in all the cases, bread prepared with the addition of the coloured wheat wholemeal (non-treated and fermented) showed higher crumb a* coordinates than control breads, and, by increasing coloured wheat wholemeal quantities in the main bread formula, bread crumb a* coordinates were increased. However, significant differences in bread crumb b* coordinates were not established. It was found that fermentation and wheat variety, as well as wheat variety × non-fermented/fermented wheat wholemeal quantity interaction significantly affected bread crumb a* coordinates (
p ≤ 0.0001,
p = 0.003, and
p = 0.0048, respectively) (
Table 9).
Colour is one of the essential attributes of the bread’s quality because consumers are more likely to accept bread with a golden-brown crust and a creamy white crumb. It has been reported that dietary fibre compounds reduce bread crust lightness by increasing brown colour, due to an oxidation reaction, more intensive caramelization, and a higher amount of the accumulated melanoids during baking [
83,
84]. However, we obtained opposite results, which could be explained by the lighter colour of non-treated and fermented purple wheat grain wholemeal and sourdough samples. Moreover, the colour of the crumb more directly reflects the ingredients used for bread making, and a smaller size of dietary fibers could cause lower differences in colour compared to that of bread without it [
85]. Similarly, in our study, the lightness of bread crumb did not differ between all bread samples. Higher values of the a* coordinate in breads prepared with the addition of coloured wheat wholemeal were due to the presence of anthocyanins and phlobaphenes in the cereal grain [
8]. Furthermore, simple sugars generated at the end of sourdough fermentation and LAB-induced release of phenolic compounds and anthocyanins could contribute to the change in colour coordinates of tested breads [
86].
Acrylamide concentration in bread samples is given in
Figure 5. Bread with coloured wheat wholemeal added showed higher concentrations of acrylamide, and, by increasing wheat wholemeal in the main bread formula, acrylamide concentration was increased. The highest acrylamide content occurred in samples prepared with 20% non-treated wheat wholemeal (8526-2 N-T-20%—123.1 µg/kg and 8529-1 N-T-20%—181.5 µg/kg). However, bread samples prepared with coloured wheat wholemeal sourdough showed significantly lower acrylamide content than breads prepared with non-treated coloured wheat wholemeal. Bread samples prepared with 8526-2 wholemeal at 5, 10, 15, and 20%, had 13.3, 32.1, 32.5, and 35.4 µg/kg lower acrylamide content, respectively. Bread samples prepared with 8526-1 wholemeal at 5, 10, 15, and 20%, had 72.3, 67.0, 66.7, and 64.6 µg/kg lower acrylamide content, respectively. Fermentation was a significant factor for acrylamide formation in bread (
p ≤ 0.0001). Significant effects on acrylamide concentration were found for the following interactions: non-fermented/fermented wheat wholemeal × wheat variety; wheat variety × non-fermented/fermented wheat wholemeal quantity; wheat variety × non-fermented/fermented wheat wholemeal quantity × non-fermented/fermented wheat wholemeal (
Table 9). However, significant correlations between the bread crust and crumb colour coordinates and acrylamide concentration were not found.
Acrylamide is an unfavorable Maillard-reaction-derived compound with a potential neurotoxic and carcinogenic effect [
87]. According to the European Commission, the set value for wheat-based bread is 80 μg/kg [
88]. High contents of asparagine and reducing sugars, as well as baking temperaturse in the range of 140–180 °C, are optimal conditions for the formation of acrylamide [
89]. Antioxidants could either inhibit or enhance acrylamide formation, but results in the literature are inconsistent [
87]. Some authors reported that the oxidized forms of antioxidants (flavonoids and phenolic acids) inhibit acrylamide formation [
77,
78,
87,
90,
91]. In this study, the acrylamide concentration of all breads made with non-treated coloured wholemeal wheat that showed antioxidant activity was higher than control breads. This could be explained by the fact that the free amino acid (e.g., asparagine) concentration in wholemeal flour is higher due to the presence of the outer layers of grain [
92]. However, the lowest content of acrylamide was found in breads made with purple wheat wholemeal sourdough, which also possessed higher DPPH-scavenging activity, compared to non-treated purple wheat. During fermentation, LAB (especially
L. plantarum) can excrete antioxidant-active compounds including active peptides and phenolics [
10]. It has been reported that a lower content of acrylamide in baked goods could be related to glucose metabolism by LAB and reduced concentration of asparagine during sourdough fermentation [
77]. This effect occurs due to the metabolism of such microorganisms as yeast and lactic acid bacteria, which utilize this amino acid for their growth [
93]. Moreover, the reduction in pH during fermentation is also important because it lowers the reactivity of free asparagine (increases protonation of the amino acid) and further inhibits the formation of Schiff base, a precursor of acrylamide [
94,
95].