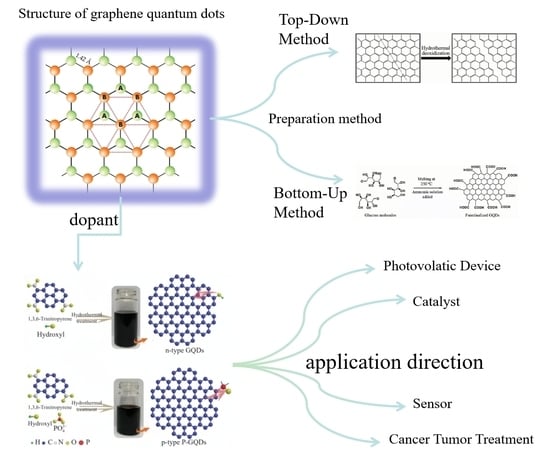
A Review of Advances in Graphene Quantum Dots: From Preparation and Modification Methods to Application
Abstract
:1. Introduction
2. Structure and Properties of Graphene Quantum Dots
2.1. Structure of Graphene Quantum Dots
2.2. Nature of Graphene Quantum Dots
2.2.1. Optical Performance
2.2.2. Water Solubility
2.2.3. Electrical Properties
2.2.4. Toxicity and Cytocompatibility
3. Preparation of Graphene Quantum Dots
3.1. Top-Down Method
3.1.1. Electrochemical Method
3.1.2. Hydrothermal Method and Solvothermal Method
3.1.3. Chemical Stripping Method
3.1.4. Microwave Ultrasonic Assisted Stripping Method
3.2. Bottom-Up Method
3.2.1. Carbonization and Pyrolysis Method
3.2.2. C60 Open Cage Method
3.2.3. Solution Chemical Method
3.2.4. Chemical Vapor Deposition Method
4. Characterization of Structural Properties of Graphene Quantum Dots
5. Modification Method of Graphene Quantum Dots
5.1. Heteroatomic Doping
5.1.1. Nitrogen Doping
5.1.2. Phosphorus Doping
5.1.3. Sulfur Doping
5.1.4. The Doping and Common Doping of Other Atoms
5.2. Surface Function
5.2.1. Hydroxyl Modification
5.2.2. Carboxyl Base Modification
5.2.3. Aromatic Ring Modification
5.2.4. Other Modifications
5.3. Composite Material
5.3.1. Undoped GQDs Composites
5.3.2. Single-Doped GQDs Composites
5.3.3. Double-Doped GQDs Composites
6. Application Field of Graphene Quantum Dots
6.1. Catalyst
6.2. Photovolatic Device
6.3. Sensor
6.3.1. Graphene Quantum-Point Photoelectric Chemical Sensor
6.3.2. Gas Sensor
6.3.3. Optical Sensor
6.4. Biological Field
6.4.1. Cancer Tumor Treatment
6.4.2. Biological Imaging
6.4.3. Drug Delivery Systems
7. The Challenges and Summary Prospects Faced
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase change materials application in battery thermal management system: A review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wang, Z.; Zhou, W.; Liu, Y.; Fan, L.; Li, Y.; Li, X. Graphene quantum dots as a smart probe for biosensing. Anal. Methods 2016, 8, 4001–4016. [Google Scholar] [CrossRef]
- Guin, S.K.; Ambolikar, A.S.; Guin, J.P.; Neogy, S. Exploring the excellent photophysical and electrochemical properties of g raphene quantum dots for complementary sensing of uranium. Sens. Actuators B Chem. 2018, 272, 559–573. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Liu, X.; Li, B.; Li, Y.; Yu, W.; et al. Surface chemistry routes to modulate the photoluminescence of graphene quantum dots: From fluorescence mechanism to up-conversion bioimaging applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Ha, M.; Kim, J.-H.; You, M.; Li, Q.; Fan, C.; Nam, J.-M. Multicomponent plasmonic nanoparticles: From heterostructured nanoparticles to colloidal composite nanostructures. Chem. Rev. 2019, 119, 12208–12278. [Google Scholar] [CrossRef]
- Yan, X.; Li, B.; Cui, X.; Wei, Q.; Tajima, K.; Li, L.-S. Independent Tuning of the Band Gap and Redox Potential of Graphene Quantum Dots. Phys. Chem. Lett. 2011, 2, 1119. [Google Scholar] [CrossRef]
- Liu, F.; Jang, M.-H.; Ha, H.D.; Kim, J.-H.; Cho, Y.-H.; Seo, T.S. Facile synthetic method for pristine graphene quantum dots and graphene oxide quantum dots: Origin of blue and green luminescence. Adv. Mater. 2013, 25, 3657–3662. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Yuan, Z.; Zhang, L.; Lin, Y.; Lu, C. Rapid screening of oxygen states in carbon quantum dots by chemiluminescence probe. Anal. Chem. 2017, 89, 12520–12526. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-transduced chemical sensors based on two-dimensional nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green. Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Chen, W.; Li, F.; Ooi, P.C.; Ye, Y.; Kim, T.W.; Guo, T. Room temperature pH-dependent ammonia gas sensors using graphene quantum dots. Sens. Actuators B 2016, 222, 763–768. [Google Scholar] [CrossRef]
- Orecchioni, M.; Menard-Moyon, C.; Delogu, L.G.; Bianco, A. Graphene and the immune system:challenges and potentiality. Adv. Drug Deliv. Rev. 2016, 105 Pt B, 163–175. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. ChemInform Abstract: Graphene Quantum Dots: Emergent Nanolights for Bioimaging, Sensors, Catalysis and Photovoltaic Devices. Chem. Commun. 2012, 48, 3686. [Google Scholar] [CrossRef]
- Coluci, V.R.; Galvao, D.S.; Baughman, R.H. Theoretical investigation of electromechanical effects for graphyne carbon nanotubes. J. Chem. Phys. 2004, 121, 3228–3237. [Google Scholar] [CrossRef]
- Geim, A.K.; MacDonald, A.H. Graphene: Exploring carbon flatland. Phys. Today 2007, 60, 35–41. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots emergent nanolights. Angew. Chem. Int. Ed. Engl. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhao, J.; Zhu, J.-J. Focusing on luminescent graphene quantum dots: Current status and future perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.-T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. Engl. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-H.; Yang, H.; Kang, D.J.; Kim, B.J. Surface engineering of graphene quantum dots and their applications as efficient surfactants. ACS Appl. Mater. Interfaces 2015, 7, 8615–8621. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tian, W.; Ren, S.; Dai, R.; Chi, Y.; Chen, G. Graphene quantum dots/L-cysteine coreactant electrochemiluminescence system and its application in sensing lead(ii) ions. ACS Appl. Mater. Interfaces 2014, 6, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tian, E.; Zhang, Y.; Mo, J. Utilizing electrostatic effect in fibrous filters for efficient airborne particles removal: Principles, fabrication, and material properties. ScienceDirect 2022, 03, 101369. [Google Scholar] [CrossRef]
- Chong, Y.; Ma, Y.; Shen, H.; Tu, X.; Zhou, X.; Xu, J.; Dai, J.; Fan, S.; Zhang, Z. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 2014, 35, 5041–5048. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Ciasca, G.; De Spirito, M.; Papi, M. Unravelling the Potential of Graphene Quantum Dots in Biomedicine and Neuroscience. Int. J. Mol. Sci. 2020, 21, 3712. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Wang, G.; Mao, J.; Liu, Z.; Sun, T.; Wang, M.; Chen, D.; Yang, Y.; Li, J.; et al. Green, rapid, and universal preparation approach of graphene quantum dots under ultraviolet irradiation. ACS Appl. Mater. Interfaces 2017, 9, 14470–14477. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, J.-X.; Wang, J.; Lim, A.; Wang, S.; Loh, K.P. One-Pot Synthesis of Fluorescent Carbon Nanoribbons, Nanoparticles, and Graphene by the Exfoliation of Graphite in Ionic Liquids. ACS Nano 2009, 3, 367–2375. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2010, 47, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Pu, Y.; Cheng, W.; Lin, S.; Shao, Z.; Liao, X. Selective, sensitive and label-free detection of Fe3+ ion in tap water using highly fluorescent graphene quantum dots. J. Fluoresc. 2019, 29, 541–548. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-T.; Wu, M.-B.; Sun, H.-D.; Xie, H.; Hu, C.; Wang, X.-N.; Wu, X.-Y.; Qiu, J.-S. Yellow-visual fluorescent carbon quantum dots from petroleum coke for the efficient detection of Cu2+ ions. New Carbon Mater. 2016, 6, 550–559. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xing, Y.; He, N.; Zhang, Y.; Lu, Z.; Zhang, J.; Zhang, Z. Preparation of graphene quantum dots for bioimaging application. J. Nanosci. Nanotechnol. 2012, 12, 2924–2948. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844. [Google Scholar] [CrossRef]
- Li, L.-L.; Ji, J.; Fei, R.; Wang, C.-Z.; Lu, Q.; Zhang, J.-R.; Jiang, L.-P.; Zhu, J.-J. A facile microwave avenue to electrochemiluminescent two-color graphene quantum dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Luo, Z.; Qi, G.; Chen, K.; Zou, M.; Yuwen, L.; Zhang, X.; Huang, W.; Wang, L. Microwave-assisted preparation of white fluorescent graphene quantum dots as a novel phosphor for enhanced white-light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2739–2744. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.-T. Upconversion and downconversion fluorescent graphene quantum dots: Ultrasonicpreparation and photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yeo, P.S.E.; Gan, C.K.; Wu, P.; Loh, K.P. Transforming C60 molecules into graphene quantum dots. Nat. Nanotechnol. 2011, 6, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xu, H.; Gan, W.; Yuan, Q. Graphene quantum dots: Recent progress in preparation and fluorescence sensing applications. RSC Adv. 2016, 6, 110775–110788. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, L.-S. Synthesis of Large, Stable Colloidal Graphene Quantum Dots with Tunable. J. Am. Chem. Soc. 2010, 132, 5944. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P. Size-Dependent Structural and Optical Characteristics of Glucose-Derived Graphene Quantum Dots. Part. Part. Syst. Charact. 2013, 30, 523–531. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Li, Q.; Yang, Y.; Wang, G.; You, X.; Mao, B.; Wang, H.; Ma, Y.; He, P.; et al. Electrochemical cutting in weak aqueous electrolyte: The strategy for efficient and controllable preparation of graphene quantum dots. Langmuir 2018, 34, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, Y.; Li, X.; Zhou, S.; Fan, L.; Yang, S. Electrochemical synthesis of small-sized red fluorescent graphene quantum dots as a bioimaging platform. Chem. Commun. 2015, 51, 2544–2546. [Google Scholar] [CrossRef]
- Wongrat, E.; Nuengnit, T.; Panyathip, R.; Chanlek, N.; Hongsith, N.; Choopun, S. Highly Selective Room Temperature Ammonia Sensors Based on ZnO Nanostructures Decorated with Graphene Quantum Dots (GQDs). Sens. Actuators B 2021, 326, 128983. [Google Scholar] [CrossRef]
- Su, J.; Zhang, X.; Tong, X.; Wang, X.; Yang, P.; Yao, F.; Guo, R.; Yuan, C. Preparation of graphene quantum dots with high quantum yield by a facile one-step method and applications for cell imaging. Mater. Lett. 2020, 271, 127806. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Zhang, J.; Li, C. One-pot hydrothermal synthesis of graphenequantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light. New J. Chem 2012, 36, 97–101. [Google Scholar] [CrossRef]
- Pan, D.; Guo, L.; Zhang, J.; Xi, C.; Xue, Q.; Huang, H.; Li, J.; Zhang, Z.; Yu, W.; Chen, Z.; et al. Cutting sp2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. Mater. Chem. 2012, 22, 3314–3318. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Chen, C.; Yang, X.; Li, C. Facile preparation and upconversion luminescence of graphene quantum dots. Chem. Commun. 2011, 47, 2580. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, Y.; Yan, X.; Cohen, M.L.; Zhang, S. Topological carbon materials: A new perspective. Phys. Rep. 2020, 868, 1–32. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, C.; Liu, D.; Wang, H.; Su, W.; Li, H.; Zhang, Y.; Yao, S. A facile and simple method for synthesis of graphene oxide quantum dots from black carbon. Green Chem. 2017, 19, 900–904. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol. Rev. 2017, 7, 157–185. [Google Scholar] [CrossRef]
- Shehab, M.; Ebrahim, S.; Soliman, M. Graphene quantum dots prepared from glucose as optical sensor for glucose. Lumin 2017, 184, 110–116. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z.; Zheng, M.; Li, J.; Zhang, Y.; Zhang, G.; Zhao, H.; Liu, X.; Xie, Z. Three colors emission froms, n co-doped graphene quantum dots for visible light H2 production and bioimaging. Adv. Opt. Mater. 2015, 3, 360–367. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Feng, X.; Mülle, K. Bottom-Up Fabrication of Photoluminescent Graphene Quantum Dots with Uniform Morphology. J. Am. Chem. Soc. 2011, 133, 15221–15223. [Google Scholar] [CrossRef]
- Kaciulis, S.; Mezzi, A.; Soltani, P.; Pizzoferrato, R.; Ciotta, E.; Prosposito, P. Graphene quantum dots obtained by unfolding fullerene. Thin Solid Film. 2019, 673, 19–25. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, M.; Lee, X.; Zhang, R.; Wang, K.; Wei, J.; Zhong, M.; Wu, D.; Zhu, H. Direct synthesis of graphene quantum dots by chemical vapor deposition. Part. Part. Syst. Charact. 2013, 30, 764–769. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.; Park, W.I.; Kim, B.-H.; Park, J.H.; Kim, T.-H.; Bong, S.; Kim, C.-H.; Chae, G.; Jun, M.; et al. Uniform Graphene Quantum Dots Patterned from Self-Assembled Silica Nanodots. Nano Lett. 2012, 12, 6078–6083. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Wang, S.; Liu, Y.; Pan, D.; Wu, M. Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem. Eng. J. 2016, 300, 75–82. [Google Scholar] [CrossRef]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green. Chem. 2018, 20, 1383–1390. [Google Scholar] [CrossRef]
- Tran, H.L.; Darmanto, W.; Doong, R.-A. Ultrasensitive detection of tetracycline using boron and nitrogen codoped graphene quantum dots from natural carbon source as the paper-based nanosensing probe in difference matrices. Nanomaterials 2020, 10, 1883. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, A.; Dharmadhikari, B.; Debnath, D.; Patra, P.; Kumar, C.V. Advances in Structural Modifications and Properties of Graphene Quantum Dots for Biomedical Applications. ACS Omega 2023, 6, 21358–21376. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Cao, Q.; Li, M.; Liu, F.; Tang, N.; Du, Y. Synthesis and photoluminescence of fluorinated graphene quantum dots. Appl. Phys. Lett. 2013, 102, 013111. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Liu, J.; Huang, K.; Chen, Y.; Li, Y.; Zhu, J.-J. Low overpotential for electrochemically reducing CO2 to CO on nitrogen-doped graphene quantum dots-wrapped single-crystalline gold nanoparticles. ACS Energy Lett. 2018, 3, 946–951. [Google Scholar] [CrossRef]
- Wang, H.; Revia, R.; Mu, Q.; Lin, G.; Yen, C.; Zhang, M. Single-layer borondoped graphene quantum dots for contrast-enhanced in vivo T1 -weighted MRI. Nanoscale Horiz. 2020, 5, 573–579. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Dega, N.K.; Tran, H.L.; Darmonto, W.; Doong, R.-A. Application of sulfur-doped graphene quantum dots@gold-carbon nanosphere for electrical pulse-induced impedimetric detection of glioma cells. Biosens. Bioelectron. 2021, 181, 113151. [Google Scholar] [CrossRef]
- Wang, H.; Mu, Q.; Wang, K.; Revia, R.A.; Yen, C.; Gu, X.; Tian, B.; Liu, J.; Zhang, M. Nitrogen and borondual-doped graphene quantum dots for near-infrared second window imaging and photothermal therapy. Appl. Mater. Today 2019, 14, 108117. [Google Scholar] [CrossRef]
- Kumara, B.N.; Kalimuthu, P.; Prasad, K.S. Synthesis, properties and potential applications of photoluminescent carbon nanoparticles: A review. Anal. Chim. Acta 2023, 1268, 341430. [Google Scholar] [CrossRef]
- Wu, W.; Zhan, L.; Fan, W.; Song, J.; Li, X.; Li, Z.; Wang, R.; Zhang, J.; Zheng, J.; Wu, M.; et al. Cu-N dopants boost electron transfer and photooxidation reactions of carbon dots. Angew. Chem. 2015, 54, 6540–6544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, W.; Han, C.; Wang, X.; Wang, Y.; Li, Z.; Wu, W.; Wu, M. Graphene structure boosts electron transfer of dual-metal doped carbon dots in photooxidation. Carbon 2018, 126, 128–134. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.-Q.; Li, N.; Zhu, J.-L.; Zhang, T.; Zhang, W.; Liu, B. Preparation of graphene quantum dots and their application in cell imaging. J. Nanomater. 2016, 2016, 9245865. [Google Scholar] [CrossRef]
- Calabro, R.L.; Yang, D.-S.; Kim, D.Y. Controlled nitrogen doping of graphene quantum dots through laser ablation in aqueous solutions for photoluminescence and electrocatalytic applications. ACS Appl. Nano Mater. 2019, 2, 6948–6959. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Xie, W.; Wang, Y.; Jia, X.; Nawaz, A.; Song, M.; Gong, J.R. Preparation of blue- and green-emissive nitrogen-doped graphene quantum dots from graphite and their application in bioimaging. Mater. Sci. Eng. C 2021, 119, 11164. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Peng, D.; Zhou, Y.; Zhang, L.; Li, D.; Tan, H.; Zhao, Z.; Xie, Z.; Sun, Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Qu, X.; Niu, Q.; Tian, C.; Fan, C.; Ye, B. A green synthesis of highly fluorescent nitrogen-doped graphene quantum dots for the highly sensitive and selective detection of mercury(II) ions and biothiols. Anal. Methods 2016, 8, 1565–1571. [Google Scholar] [CrossRef]
- Benitez-Martinez, S.; Lopez-Lorente, A.I.; Valcarcel, M. Determination of TiO2 nanoparticles in sunscreen using N-doped graphene quantum dots as a fluorescent probe. Microchim. Acta 2016, 183, 781–789. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, B.; Liu, Z.; Chen, D.; Zhao, Y.; Guo, Q.; Wang, G. Hydrothermal synthesis of N, P co-doped graphene quantum dots for high-performance Fe3+ detection and bioimaging. J. Nanopart. Res. 2021, 23, 40. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, Y.; Wang, J.; Liu, X.; Wang, L.; Liu, X.; Xue, W.; Ma, N. Electrochemical synthesis of phosphorus doped graphene quantum dots for free radical scavenging. Phys. Chem. Chem. Phys. 2017, 19, 11631–11638. [Google Scholar] [CrossRef]
- Wang, W.; Xu, S.; Li, N.; Huang, Z.; Su, B.; Chen, X. Sulfur and phosphorus codoped graphene quantum dots for fluorescent monitoring of nitrite in pickles. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2019, 221, 117211. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Shen, C.; Yan, J.; Xi, F.; Dong, X.; Liu, J. Tailoring the electronic properties of graphene quantum dots by P doping and its enhanced performance in metal-free composite photocatalyst. J. Phys. Chem. C 2018, 122, 349–358. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, G.; Jian, L.; Murtaza, I.; Meng, H.; Liu, Y.; Min, Y. Facile synthesis of defect-rich nitrogen and sulfur co-doped graphene quantum dots as metal-free electrocatalyst for the oxygen reduction reaction. J. Alloys Compd. 2019, 792, 844–850. [Google Scholar] [CrossRef]
- Yang, L.; Wei, J.; Ma, Z.; Song, P.; Ma, J.; Zhao, Y.; Huang, Z.; Zhang, M.; Yang, F. The fabrication of micro/nano structures by laser machining. Nanomaterials 2019, 9, 1789. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, M.; Sun, L.; Xu, Y.; Li, M.; Hu, G.; Tang, T.; Wen, J.; Li, X.; Zhang, J.; et al. High fluorescent sulfur regulating graphene quantum dots with tunable photoluminescence properties. J. Colloid Interface Sci. 2018, 529, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.T.; Gonzalez-Rodriguez, R.; Ryan, C.; Faerber, N.; Cofffer, J.L.; Anton; Naumov, V. Photo-and electroluminescence from nitrogen-doped and nitrogensulfur codoped graphene quantum dots. Adv. Funct. Mater. 2018, 28, 1804337. [Google Scholar] [CrossRef]
- Luo, Y.; Li, M.; Hu, G.; Tang, T.; Wen, J.; Li, X.; Wang, L. Enhanced photocatalytic activity of sulfur-doped graphene quantum dots decorated with TiO2 nanocomposites. Mater. Res. Bull. 2018, 97, 428–435. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Ge, S.; He, J.; Ma, C.; Liu, J.; Xi, F.; Dong, X. One-step synthesis of boron-doped graphene quantum dots for fluorescent sensors and biosensor. Talanta 2019, 199, 581–589. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.-Y.; Liang, R.-P.; Li, Y.-H.; Qiu, J.-D. Boron-doped graphene quantum dots for selective glucose sensing based on the “abnormal” aggregation-induced photoluminescence enhancement. Anal. Chem. 2014, 86, 4423–4430. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, S.; Hou, X.; Sun, Z.; Jiang, Y.; Dong, Z.; Tao, Q.; Man, J.; Cao, Y. Coal-derived nitrogen, phosphorus and sulfur co-doped graphene quantum dots: A promising ion fluorescent probe. Appl. Surf. Sci. 2018, 445, 519–526. [Google Scholar] [CrossRef]
- Islam, M.S.; Deng, Y.; Tong, L.; Roy, A.K.; Faisal, S.N.; Hassan, M.; Minnett, A.I.; Gomes, V.G. In-situ direct grafting of graphene quantum dots onto carbon fibre by low temperature chemical synthesis for high performance flexible fabric supercapacitor. Mater. Today Commun. 2016, 10, 112–119. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Yin, H.; Zhang, K.-Q.; Chen, X.; Wang, K.-Z. Chemosensing properties and logic gate behaviors of graphene quantum dotappended terpyridine. Mater. Sci. Eng. C 2019, 99, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Pourhashem, S.; Ghasemy, E.; Rashidi, A.; Vaezi, M.R. Corrosion protection properties of novel epoxy nanocomposite coatings containing silane functionalized graphenequantum dots. J. Alloys Compd. 2018, 731, 1112–1118. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, W.; Zhong, M.; Zhang, J.; Guo, S. Carbon nanofibers cross-linked and decorated with graphene quantum dots as binder-free electrodes for flexible supercapacitors. J. Phys. Chem. C 2021, 125, 143–151. [Google Scholar] [CrossRef]
- Chan, D.K.L.; Cheung, P.L.; Yu, J.C. A visible-light-driven composite photocatalyst of TiO2 nanotube arrays and graphene quantum dots. Beilstein J. Nanotechnol. 2014, 5, 689–695. [Google Scholar] [CrossRef]
- Tachi, S.; Morita, H.; Takahashi, M.; Okabayashi, Y.; Hosokai, T.; Sugai, T.; Kuwahara, S. Quantum yield enhancement in graphene quantum dots via esterifcation with benzyl alcohol. Sci. Rep. 2019, 9, 14115. [Google Scholar] [CrossRef]
- Luo, P.; Guan, X.; Yu, Y.; Li, X. 4-Methoxyphenyl grafted onto graphene quantum dots surface via diazonium chemistry method. Opt. Quantum Electron. 2018, 50, 269. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Kong, W.; Wang, L.; Li, Y. Post-epoxidation of graphene quantum dots. Chem. Phys. Lett. 2018, 706, 140–144. [Google Scholar] [CrossRef]
- Hossain, Z.; Shimizu, N. In Situ functionalization of graphene with reactive end group through amine diazotiz-ation. J. Phys. Chem. C 2017, 121, 25223–25228. [Google Scholar] [CrossRef]
- Ge, Q.; Kong, W.-H.; Liu, X.-Q.; Wang, Y.-M.; Wang, L.-F.; Ma, N.; Li, Y. Hydroxylated graphene quantum dots as fluorescent probes for sensitive detec-tion of metal ions. Int. J. Miner. Metall. Mater. 2020, 27, 91–99. [Google Scholar] [CrossRef]
- Ashraf, G.; Asif, M.; Aziz, A.; Dao, A.Q.; Zhang, T.; Iftikhar, T.; Wang, Q.; Liu, H. Facet-energy inspired metal oxide extended hexapods decorated with graphene quantum dots: Sensitive detection of bisphenol A in live cells. Nanoscale 2020, 12, 9014–9023. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.; Lau, S. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- He, R.; Chen, R.; Luo, J.; Zhang, S.; Xu, D. Fabrication of graphene quantum dots modified BiOI/PAN flexible fiber with en-hanced photocatalytic activity. Acta Phys. Chim. Sin. 2021, 37, 2011022. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Yang, Y.; Guo, S.; Zhang, J. Composites of graphene quantum dots and reduced graphene oxide as catalysts for nitroarene reduction. ACS Omega 2017, 2, 7293–7298. [Google Scholar] [CrossRef]
- Lalit, G.; Neha, A.; Rajni, V.; Bishnoi, S.; Husale, S.; Pandey, R.; Gupta, G. Graphene quantum dot-sensitized ZnO-nanorod/GaN-nanotower heterostructure-based high-performance UV photodetectors. ACS Appl. Mater. Interfaces 2020, 12, 47038–47047. [Google Scholar] [CrossRef]
- Yang, H.; Wang, P.; Wang, D.; Zhu, Y.; Xie, K.; Zhao, X.; Yang, J.; Wang, X. New understanding on photocatalytic mechanism of Nitrogen-doped graphene quantum dots-decorated BiVO4 nanojunction photocatalysts. ACS Omega 2017, 2, 3766–3773. [Google Scholar] [CrossRef]
- Li, F.; Li, M.; Luo, Y.; Li, M.; Li, X.; Zhang, J.; Wang, L. The synergistic effect of pyridinic nitrogen and graphitic nitrogen of nitrogen-doped graphene quantum dots for enhanced TiO2 nanocomposites photocatalytic performance. Catalysts 2018, 8, 438–449. [Google Scholar] [CrossRef]
- Li, K.; Ji, M.; Chen, R.; Jiang, Q.; Xia, J.; Li, H. Construction of nitrogen and phosphorus co-doped graphene quantum dots/Bi5O7 I composites for accelerated charge separation and enhanced photocatalytic degradation performance. Chin. J. Catal. 2020, 41, 1230–1239. [Google Scholar] [CrossRef]
- Cai, A.; Wang, Q.; Chang, Y.; Wang, X. Graphitic carbon nitride decorated with S, N co-doped graphene quantum dots for enhanced visible-light-driven photocatalysis. J. Alloys Compd. 2017, 692, 189–193. [Google Scholar] [CrossRef]
- Tian, H.; Shen, K.; Hu, X.; Qiao, L.; Zheng, W. N, S co-doped graphene quantum dots-graphene-TiO2 nanotubes composite with enhanced photocatalytic activity. J. Alloys Compd. 2017, 691, 369–377. [Google Scholar] [CrossRef]
- Li, N.; Than, A.; Wang, X.; Xu, S.; Sun, L.; Duan, H.; Xu, C.; Chen, P. Ultrasensitive profiling of metabolites using tyramine-functionalized graphene quantum dots. ACS Nano 2016, 10, 3622–3629. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Z.; Wang, T.; Ma, C.; Yan, Y. N-doped graphene quantum dots for enhancing multi-level Bi2Ti2O7 spheres photocatalytic activity via electronic trapping. J. Dispers. Sci. Technol. 2022, 43, 639–648. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Liu, Q.; Chen, W.; Yin, Y.; Ge, L.; Li, H.; Wang, K. Boosting the visible-lightphotoactivity of BiOCl/BiVO4/N-GQDs ternary hetero-junctions based on internal Z-scheme charge transfer of N-GQDs: Simultaneous band gap narrowing and carrierlifetime prolonging. ACS Appl. Mater. Interfaces 2017, 9, 38832–38841. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.H.; Hu, Y.; Shi, G.Q.; Dai, L.; Qu, L.T. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.Q.; Deng, L.; Hou, Y.B.; Qu, L.T. An Electrochemical Avenue to Green-Luminescent Graphene Quantum Dots as Potential Electron-Acceptors for Photovoltaics. Adv. Mater. 2011, 23, 776. [Google Scholar] [CrossRef]
- Gupta, V.; Chaudhary, N.; Srivastava, R.; Sharma, G.D.; Bhardwaj, R.; Chand, S. Luminscent Graphene Quantum Dots for Organic Photovoltaic Devices. J. Am. Chem. Soc. 2011, 133, 9960–9963. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, G.; Zhu, L.; Li, G. Graphene quantum dots-based platform for the fabrication of electrochemical biosensors. Electrochem. Commun. 2011, 13, 31–33. [Google Scholar] [CrossRef]
- Ran, Z.; Yang, H.; Li, Z.; Wang, K.; Zhao, J.; Ran, X.; Du, G.; Yang, L. Pillar[6]arene@AuNPs functionalized N-CQDs@Co3O4 hybrid composite for ultrasensitive electrochemical detection of human epididymis protein 4. ACS Sustain. Chem. Eng. 2020, 8, 10161–10172. [Google Scholar] [CrossRef]
- Zhang, P.; Zhuo, Y.; Chang, Y.; Yuan, R.; Chai, Y. Electrochemiluminescent g raphene quantum dots as a sensing platform: A dual amplification for microRNA assay. Anal. Chem. 2015, 87, 10385–10391. [Google Scholar] [CrossRef]
- Wang, M.; Yin, H.; Zhou, Y.; Meng, X.; Waterhouse, G.I.N.; Ai, S. A novel photoelectro chemical biosensor for the sensitive detection of dual microRNAs using molybdenum carbide nanotubes as nanocarriers and energy transfer between CQDs and AuNPs. Chem. Eng. J. 2019, 365, 351–357. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, Y.; Wu, H.; Chen, C.; Chi, Y.; Chen, G. Electrochemiluminescence sensor for hexavalent chromium based on the g raphene quantum dots/peroxodisulfate system. Electrochim. Acta 2015, 151, 552–557. [Google Scholar] [CrossRef]
- Qu, Z.; Na, W.; Liu, X.; Liu, H.; Su, X. A novel fluorescence biosensor for sensitivity detection of tyrosinase. Anal. Chim. Acta 2018, 997, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Ji, J.; Sun, J.; Wang, J.; Zhang, Y.; Ding, H.; Lu, Y.; Xu, D.; Sun, X. A novel magnetic fluorescent biosensor based on graphene quantum dots for rapid, efficient, and sensitive separation and detection of circulating tumor cells. Anal. Bioanal. Chem. 2019, 411, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-K.; Li, Y.-Y.; Yao, H.-C.; Li, Z.-J. Nitrogen-doped graphene quantum dots-modified mesoporous SnO2 hierarchical hollow cubes for low temperature detection of nitrogen dioxide. Actuators B 2021, 339, 129882. [Google Scholar] [CrossRef]
- Hakimi, M.; Salehi, A.; Boroumand, F.A.; Mosleh, N. Fabrication of a Room Temperature Ammonia Gas Sensor Based on Polyaniline with N-doped Graphene Quantum Dots. IEEE Sens. J. 2018, 18, 2245–2252. [Google Scholar] [CrossRef]
- Parvizi, R.; Azad, S.; Dashtian, K.; Ghaedi, M.; Heidari, H. Natural Source-Based Graphene as Sensitising Agents for Air Quality Monitoring. Sci. Rep. 2019, 9, 3798. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Xu, L.; Zhu, S.; Yang, S.; Chen, X.; Dong, B.; Bai, X.; Lu, G.; Song, H. Graphene quantum dot-functionalized three-dimensional ordered mesoporous ZnO for acetone detection toward diagnosis of diabetes. Nanoscale 2019, 11, 11496–11504. [Google Scholar] [CrossRef]
- Arunragsa, S.; Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Hydroxyl edge-functionalized Graphene quantum dots for gas-sensing applications. Diam. Relat. Mater. 2020, 105, 107790. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Hasani, A.; Nouri, M.; Mahyarii, M.; Salehii, A. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens. Actuators B 2016, 229, 239–248. [Google Scholar] [CrossRef]
- Hakimi, M.; Salehi, A.; Boroumand, F. Fabrication and Characterization of an Ammonia Gas Sensor Based on PEDOT-PSS with N-doped Graphene Quantum Dots Dopant. IEEE Sens. J. 2016, 16, 6149–6154. [Google Scholar] [CrossRef]
- Abbasabadi, M.K.; Zand, H.R.E.; Khodabakhshi, S.; Gholami, P.; Rashidi, A. Synthesis of new functionalized reduced graphene oxide quantum dot composite for high-performance NO2 gas sensor. Res. Chem. Intermed. 2021, 47, 2279–2296. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Zhou, R.-H.; Pan, Y.-P.; Yao, H.-C.; Li, Z. N-Doped graphene quantum dots-decorated three-dimensional ordered macroporous In2O3 for NO2 sensing at low temperatures. ACS Appl. Mater. Interfaces 2020, 12, 34245–34253. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Yang, Y.; Tang, R.; Sun, Y.; Sun, S.; Cao, X.; Li, P.; Zhang, Z.; Li, X. One-step hydrothermal synthesis of a flexible nanopaper-based Fe3+ sensor using carbon quantum dot grafted cellulose nanofibrils. Cellulose 2020, 27, 729–742. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Li, X.; Zhao, B.; Wen, X.; Zhang, G.; Dong, C.; Shuang, S. Controllable synthesis of green and blue fluorescent carbon nanodots for pH and Cu(2+) sensing in living cells. Biosens. Bioelectron. 2016, 77, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, K.; Liu, Q.; Liu, Y.; Xie, M. Visual and fluorescent detection of mercury ions by using a dually emissive ratiometric nanohybrid containing carbon dots and CdTe quantum dots. Microchim. Acta 2017, 184, 1199–1206. [Google Scholar] [CrossRef]
- Das, R.; Sugimoto, H.; Fujii, M.; Giri, P.K. Quantitative understanding of charge-transfer-mediated Fe3+ sensing and fast photoresponse by N-doped graphene quantum dots decorated on plasmonic Au nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4755–4768. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Ju, J.; Regmi, S.; Fu, A.; Lim, S.; Liu, Q. Graphene quantum dot based chargereversal nanomaterial for nucleus-targeted drug delivery and efficiency controllable photodynamic therapy. Biophotonics 2019, 12, e201800367. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Tang, B.; Yuan, B.; Sun, L.; Wang, X.-G. A review of optical imaging and therapy using nanosized graphene and graphene oxide. Biomaterials 2013, 34, 9519–9534. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, H.; Yang, Z.; Zhong, X.; Chen, Y.; Dai, W.; Zhang, X. Aptamer-conjugated graphenequantum dots/porphyrin derivative theranostic agent for intracellular cancer-related microRNA detection and fluorescence-guided photothermal/photodynamic synergetic therapy. ACS Appl. Mater. Interfaces 2017, 9, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tian, Z.; Liu, J.; Zhu, Y.; Hanagata, N. Mesoporous silica nanoparticles capped with graphene quantum dots for potential chemo-photothermal synergistic cancer therapy. Langmuir 2017, 33, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Gao, H.; Lai, L.; Pang, Y.; Zheng, S.; Niu, Y.; Li, X. Preparation of highly fluorescent sulfur doped graphene quantum dots for live cell imaging. J. Lumin. 2018, 197, 147–152. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Shen, X.-C.; Chang, C.-Y.; Kao, H.-F.; Lin, S.-H.; Wang, J.-Y.; Wu, P.-C. Multiplexed graphene quantum dots with excitation-wavelength-independent photoluminescence, as two-photon probes, and in ultraviolet-near Infrared bioimaging. ACS Nano 2020, 14, 11502–11509. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Gao, W.; Tang, B. Recent advancement in graphene quantum dots based fluorescent sensor: Design, construction and bio-medical applications. Coord. Chem. Rev. 2023, 478, 214966. [Google Scholar] [CrossRef]
- Zhao, X.-E.; Lei, C.; Gao, Y.; Gao, H.; Zhu, S.; Yang, X.; You, J.; Wang, H. A ratiometric fluorescent nanosensor for the detection of silver ions using graphene quantum dots. Sens. Actuators B Chem. 2017, 253, 239–246. [Google Scholar] [CrossRef]
- Liu, Z.; Mo, Z.; Niu, X.; Yang, X.; Jiang, Y.; Zhao, P.; Liu, N.; Guo, R. Highly sensitive fluorescence sensor for mercury(II) based on boron- and nitrogen-co-doped graphene quantum dots. J. Colloid Interface Sci. 2020, 566, 357–368. [Google Scholar] [CrossRef]
- Xu, A.; He, P.; Ye, C.; Liu, Z.; Gu, B.; Gao, B.; Li, Y.; Dong, H.; Chen, D.; Wang, G.; et al. Polarizing graphene quantum dots toward long-acting intracellular reactive oxygen species evaluation and tumor detection. ACS Appl. Mater. Interfaces 2020, 12, 10781–10790. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhao, D.; Liu, Y.; Guo, X.; Zhang, H.; Zhang, L.; Zheng, C. Chemoembolization of liver cancer with doxorubicin-loaded CalliSpheres microspheres: Plasma pharmacokinetics, intratumoral drug concentration, and tumor necrosis in a rabbit model. Drug Deliv. Transl. Res. 2020, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Tasciotti, E.; Liu, X.; Bhavane, R.; Plant, K.; Leonard, A.D.; Price, B.K.; Cheng, M.M.-C.; Decuzzl, P.; Tour, J.M.; Robertson, F.; et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat. Nanotechnol. 2008, 3, 151–157. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Zhou, X.; Han, T.; Xin, X.; Wu, J.; Zhang, J.; Guo, S. Enhancing cell nucleus accumulation and DNA cleavage activity of anti-cancer drug via graphene quantum dots. Sci. Rep. 2013, 3, 2852. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S.; Romeo, R.; Giofré, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Su, Y.-L.; Cheng, W.; Hu, P.-F.; Chiang, C.-S.; Chen, W.-T.; Hu, S.-H. Graphene quantum dots-mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 2019, 19, 69–81. [Google Scholar] [CrossRef]



























| Methods | Advantages | Disadvantages | Refs. | |
|---|---|---|---|---|
| Top-down | Hydrothermal synthesis; Solvothermal synthesis | Large output; Simple operation | Irregularly size and shape; Strong reductants are needed | [24,36,37] |
| Acidic oxidation | Large output; Simple operation | There are lots of oxygen-containing functional groups left on the GQD surface; Numerous defects | [38] | |
| Oxidation cutting | Relatively cheap raw materials; Large output; Simple operation | Too much oxygen—containing functional groups | [39] | |
| Ultrasonic exfoliation | Simple operation; No reduction process; Fewer surface defects and more stable electronic properties | Depending on the quality of the carbon fiber, Special devices are needed | [39,40,41] |
| Methods | Advantages | Disadvantages | Refs. | |
|---|---|---|---|---|
| Down-top | Carbonization of organic molecules | Well-defined, monodispersed structures; Simple operation | Low-output | [43,44] |
| C60 open cage method | Well-defined monodispersed structures; Precise control over both size and shape | Strict reaction conditions; Very high heating temperature; Expensive raw materials | [44] | |
| Solution chemical method | Well-defined monodispersed structures; Easy control of both size and shape; High purity | Difficulty preventing aggregation; Low-output | [44,45] |
| Sensing Materials | Temperature | Gas Concentration (ppm) | Sensitivity (%) | Response/Recovery Time | Ref. |
|---|---|---|---|---|---|
| GQDs | Room temperature | 10~400 | 14.9~44.5 | 26/21 s | [16] |
| OH-GQDs | Room temperature | 10~500 | 1.54~76.63 | 64/69 s | [130] |
| S,N:GQDs/PANI | Room temperature | 100~1000 | 42~385 | 115/44 s | [131] |
| N-GQDs/PEDOT-PSS | Room temperature | 1500 | 212.32 | 6.8/9 min | [132] |
| N-GQDs/PANI | Room temperature | 1500 | 110.92 | 7.0/0.083 min | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Liu, L.; Shi, M.; Wang, Y.; Meng, X.; Chen, Y.; Huang, Q.; Liu, C. A Review of Advances in Graphene Quantum Dots: From Preparation and Modification Methods to Application. C 2024, 10, 7. https://doi.org/10.3390/c10010007
Cui Y, Liu L, Shi M, Wang Y, Meng X, Chen Y, Huang Q, Liu C. A Review of Advances in Graphene Quantum Dots: From Preparation and Modification Methods to Application. C. 2024; 10(1):7. https://doi.org/10.3390/c10010007
Chicago/Turabian StyleCui, Yibo, Luoyi Liu, Mengna Shi, Yuhao Wang, Xiaokai Meng, Yanjun Chen, Que Huang, and Changcheng Liu. 2024. "A Review of Advances in Graphene Quantum Dots: From Preparation and Modification Methods to Application" C 10, no. 1: 7. https://doi.org/10.3390/c10010007