Injectable Gamboge-Based In Situ Gel for Sustained Delivery of Imatinib Mesylate
Abstract
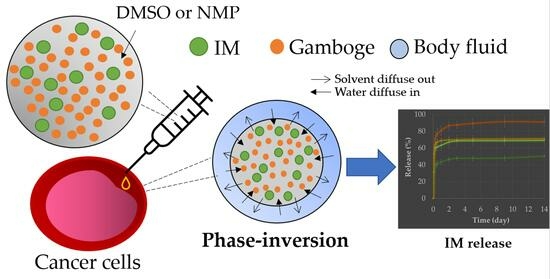
:1. Introduction
2. Results and Discussion
2.1. Gamboge-Based ISGs
2.1.1. Gel Formation
2.1.2. pH, Viscosity, and Injectability
2.2. Imatinib-Loaded 30% w/w Gamboge-Based ISGs
2.2.1. Gel Formation
2.2.2. Interfacial Interaction
2.2.3. Rheological Property of IM-Loaded 30% w/w Gamboge ISG
2.2.4. Injectability and Mechanical Property
2.2.5. In Vitro Drug Release Study
2.2.6. Quantification of GA
2.2.7. GA Release
2.2.8. SEM Morphology Study
2.2.9. Cytotoxicity Activity
Gamboge Resin Cytotoxicity
IM-Loaded Gamboge-Based ISG Cytotoxicity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Gamboge ISG
4.3. Physicochemical Study
4.3.1. Rheological Behavior
4.3.2. Injectability
4.3.3. Mechanical Property
4.4. Gel Formation
4.5. In Vitro Drug Release
4.6. SEM Image Analysis
4.7. Cytotoxicity Test of Gamboge and ISGs
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Che Hassan, N.K.N.; Taher, M.; Susanti, D. Phytochemical Constituents and Pharmacological Properties of Garcinia Xanthochymus—A Review. Biomed. Pharmacother. 2018, 106, 1378–1389. [Google Scholar] [CrossRef]
- Han, Q.-B.; Qiao, C.-F.; Song, J.-Z.; Yang, N.-Y.; Cao, X.-W.; Peng, Y.; Yang, D.-J.; Chen, S.-L.; Xu, H.-X. Cytotoxic Prenylated Phenolic Compounds from the Twig Bark of Garcinia Xanthochymus. Chem. Biodivers. 2007, 4, 940–946. [Google Scholar] [CrossRef]
- Chen, Y.; He, S.; Tang, C.; Li, J.; Yang, G. Caged Polyprenylated Xanthones from the Resin of Garcinia hanburyi. Fitoterapia 2016, 109, 106–112. [Google Scholar] [CrossRef]
- Anantachoke, N.; Tuchinda, P.; Kuhakarn, C.; Pohmakotr, M.; Reutrakul, V. Prenylated Caged Xanthones: Chemistry and Biology. Pharm. Biol. 2012, 50, 78–91. [Google Scholar] [CrossRef]
- Wang, X.; Lu, N.; Yang, Q.; Gong, D.; Lin, C.; Zhang, S.; Xi, M.; Gao, Y.; Wei, L.; Guo, Q.; et al. Studies on Chemical Modification and Biology of a Natural Product, Gambogic Acid (III): Determination of the Essential Pharmacophore for Biological Activity. Eur. J. Med. Chem. 2011, 46, 1280–1290. [Google Scholar] [CrossRef]
- Garg, G.; Khandelwal, A.; Blagg, B.S.J. Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects. In Advances in Cancer Research; Isaacs, J., Whitesell, L.B.T.-A., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 129, pp. 51–88. ISBN 0065-230X. [Google Scholar]
- Xu, B.; Ding, J.; Chen, K.-X.; Miao, Z.-H.; Huang, H.; Liu, H.; Luo, X.-M. Advances in Cancer Chemotherapeutic Drug Research in China. In Recent Advances in Cancer Research and Therapy; Liu, X.-Y., Pestka, S., Shi, Y.-F., Eds.; Elsevier: Oxford, UK, 2012; pp. 287–350. ISBN 978-0-12-397833-2. [Google Scholar]
- Chi, Y.; Zhan, X.; Yu, H.; Xie, G.; Wang, Z.; Xiao, W.; Wang, Y.; Xiong, F.; Hu, J.; Yang, L.; et al. An Open-Labeled, Randomized, Multicenter Phase IIa Study of Gambogic Acid Injection for Advanced Malignant Tumors. Chin. Med. J. 2013, 126, 1642–1646. [Google Scholar]
- Zhu, W.; Wang, R.; Liu, F.; Zhang, Z.; Huang, X.; Zhu, J.; Feng, F.; Liu, W.; Qu, W. Construction of Long Circulating and Deep Tumor Penetrating Gambogic Acid-Hydroxyethyl Starch Nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 77, 103910. [Google Scholar] [CrossRef]
- Sweetman, S.C. Martindale: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Al-Hadiya, B.M.H.; Bakheit, A.H.H.; Abd-Elgalil, A.A. Imatinib Mesylate. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2014; Volume 39, pp. 265–297. ISBN 1871-5125. [Google Scholar]
- Deininger, M.; Buchdunger, E.; Druker, B.J. The Development of Imatinib as a Therapeutic Agent for Chronic Myeloid Leukemia. Blood 2005, 105, 2640–2653. [Google Scholar] [CrossRef]
- Ben Ami, E.; Demetri, G.D. A Safety Evaluation of Imatinib Mesylate in the Treatment of Gastrointestinal Stromal Tumor. Expert Opin. Drug Saf. 2016, 15, 571–578. [Google Scholar] [CrossRef]
- Yust-Katz, S.; Khagi, S.; Gilbert, M.R. Neurologic Complications. In Abeloff’s Clinical Oncology, 6th ed.; Niederhuber, J.E., Armitage, J.O., Kastan, M.B., Doroshow, J.H., Tepper, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 688–706.e7. ISBN 978-0-323-47674-4. [Google Scholar]
- Aronson, J.K. Imatinib. In Meyler’s Side Effects of Drugs; Elsevier: Amsterdam, The Netherlands, 2016; pp. 22–24. [Google Scholar]
- Gong, D.; Celi, N.; Zhang, D.; Cai, J. Magnetic Biohybrid Microrobot Multimers Based on Chlorella Cells for Enhanced Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2022, 14, 6320–6330. [Google Scholar] [CrossRef]
- Kouchak, M. In Situ Gelling Systems for Drug Delivery. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e20126. [Google Scholar] [CrossRef]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in Situ Implants Formed by Phase Inversion: Critical Physicochemical Parameters to Modulate Drug Release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef]
- Kanwar, N.; Sinha, V.R. In Situ Forming Depot as Sustained-Release Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 93–136. [Google Scholar] [CrossRef]
- Thakur, R.R.S.; McMillan, H.L.; Jones, D.S. Solvent Induced Phase Inversion-Based in Situ Forming Controlled Release Drug Delivery Implants. J. Control. Release 2014, 176, 8–23. [Google Scholar] [CrossRef]
- Karp, F.; Turino, L.N.; Helbling, I.M.; Islan, G.A.; Luna, J.A.; Estenoz, D.A. In Situ Formed Implants, Based on PLGA and Eudragit Blends, for Novel Florfenicol Controlled Release Formulations. J. Pharm. Sci. 2021, 110, 1270–1278. [Google Scholar] [CrossRef]
- Ganguly, S.; Dash, A.K. A Novel in Situ Gel for Sustained Drug Delivery and Targeting. Int. J. Pharm. 2004, 276, 83–92. [Google Scholar] [CrossRef]
- Ravivarapu, H.B.; Moyer, K.L.; Dunn, R.L. Sustained Activity and Release of Leuprolide Acetate from an in Situ Forming Polymeric Implant. AAPS PharmSciTech 2015, 1, 1. [Google Scholar] [CrossRef]
- Cismowski, M.J. Imatinib. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–7. ISBN 978-0-08-055232-3. [Google Scholar]
- Solorio, L.; Babin, B.M.; Patel, R.B.; Mach, J.; Azar, N.; Exner, A.A. Noninvasive Characterization of in Situ Forming Implants Using Diagnostic Ultrasound. J. Control. Release 2010, 143, 183–190. [Google Scholar] [CrossRef]
- Khaing, E.M.; Chantadee, T.; Intaraphairot, T.; Phaechamud, T. Gamboge Resin-Based Phase Separation In Situ Forming Gel. Key Eng. Mater. 2019, 819, 215–220. [Google Scholar] [CrossRef]
- Masomian, M.; Rahman, R.N.Z.R.A.; Salleh, A.B. A Novel Method of Affinity Tag Cleavage in the Purification of a Recombinant Thermostable Lipase from Aneurinibacillus Thermoaerophilus Strain HZ. Catalysts 2018, 8, 479. [Google Scholar] [CrossRef]
- Zare, M.; Mobedi, H.; Barzin, J.; Mivehchi, H.; Jamshidi, A.; Mashayekhi, R. Effect of Additives on Release Profile of Leuprolide Acetate in an in Situ Forming Controlled-Release System: In Vitro Study. J. Appl. Polym. Sci. 2008, 107, 3781–3787. [Google Scholar] [CrossRef]
- Phaechamud, T.; Setthajindalert, O. Antimicrobial In-Situ Forming Gels Based on Bleached Shellac and Different Solvents. J. Drug Deliv. Sci. Technol. 2018, 46, 285–293. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Mahadlek, J.; Okonogi, S.; Pichayakorn, W.; Phaechamud, T. Imatinib Mesylate-Loaded Rosin/Cinnamon Oil-Based In Situ Forming Gel against Colorectal Cancer Cells. Gels 2022, 8, 526. [Google Scholar] [CrossRef]
- Xu, M.; Tan, H.; Fu, W.; Pan, L.; Tang, Y.; Huang, S.; Xiu, Y. Simultaneous Determination of Seven Components in Gamboge and Its Processed Products Using a Single Reference Standard. Chin. Herb. Med. 2017, 9, 42–49. [Google Scholar] [CrossRef]
- Perumal, M.; Balraj, A.; Jayaraman, D.; Krishnan, J. Experimental Investigation of Density, Viscosity, and Surface Tension of Aqueous Tetrabutylammonium-Based Ionic Liquids. Environ. Sci. Pollut. Res. 2021, 28, 63599–63613. [Google Scholar] [CrossRef]
- Rungseevijitprapa, W.; Bodmeier, R. Injectability of Biodegradable in Situ Forming Microparticle Systems (ISM). Eur. J. Pharm. Sci. 2009, 36, 524–531. [Google Scholar] [CrossRef]
- Velpandian, T.; Mathur, R.; Agarwal, N.K.; Arora, B.; Kumar, L.; Gupta, S.K. Development and Validation of a Simple Liquid Chromatographic Method with Ultraviolet Detection for the Determination of Imatinib in Biological Samples. J. Chromatogr. B 2004, 804, 431–434. [Google Scholar] [CrossRef]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Phaechamud, T. Saturated Fatty Acid-Based In Situ Forming Matrices for Localized Antimicrobial Delivery. Pharmaceutics 2020, 12, 808. [Google Scholar] [CrossRef]
- Chhabra, R.P. Non-Newtonian Fluids: An Introduction. In Rheology of Complex Fluids; Krishnan, J.M., Deshpande, A.P., Kumar, P.B.S., Eds.; Springer: New York, NY, USA, 2010; pp. 3–34. ISBN 978-1-4419-6494-6. [Google Scholar]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-Based Antisolvent-Induced in Situ Forming Matrix for Crevicular Pocket Delivery of Vancomycin Hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Galmarini, M.V.; Baeza, R.; Sanchez, V.; Zamora, M.C.; Chirife, J. Comparison of the Viscosity of Trehalose and Sucrose Solutions at Various Temperatures: Effect of Guar Gum Addition. LWT-Food Sci. Technol. 2011, 44, 186–190. [Google Scholar] [CrossRef]
- Sheshala, R.; Hong, G.C.; Yee, W.P.; Meka, V.S.; Thakur, R.R.S. In Situ Forming Phase-Inversion Implants for Sustained Ocular Delivery of Triamcinolone Acetonide. Drug Deliv. Transl. Res. 2019, 9, 534–542. [Google Scholar] [CrossRef]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-Loaded Solvent Exchange-Induced in Situ Forming Beta-Cyclodextrin Gel and Microparticle for Periodontal Pocket Delivery. Mater. Sci. Eng. C 2020, 117, 111275. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Natural-Resin in-Situ-Forming Gels: Physicochemical Characteristics and Bioactivities. Pharm. Sci. Asia 2021, 48, 461–470. [Google Scholar] [CrossRef]
- Nasongkla, N.; Nittayacharn, P.; Rotjanasitthikit, A.; Pungbangkadee, K.; Manaspon, C. Paclitaxel-Loaded Polymeric Depots as Injectable Drug Delivery System for Cancer Chemotherapy of Hepatocellular Carcinoma. Pharm. Dev. Technol. 2017, 22, 652–658. [Google Scholar] [CrossRef]
- Liu, H.; Venkatraman, S.S. Cosolvent Effects on the Drug Release and Depot Swelling in Injectable In Situ Depot-Forming Systems. J. Pharm. Sci. 2012, 101, 1783–1793. [Google Scholar] [CrossRef]
- Xu, H.; Ji, H.; Li, Z.; Qiao, W.; Wang, C.; Tang, J. In Vivo Pharmacokinetics and in Vitro Release of Imatinib Mesylate-Loaded Liposomes for Pulmonary Delivery. Int. J. Nanomed. 2021, 16, 1221–1229. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Bruschi, M. Strategies to Modify the Drug Release from Pharmaceutical Systems, 1st ed.; Woodhead Publishing Limited: Sawston, UK, 2015. [Google Scholar]
- Khaing, E.M.; Jitrangsri, K.; Mahadlek, J.; Sukonpan, C.; Phaechamud, T. The Validation HPLC Method for Determination of Gambogic Acid in Gamboge Resin. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1234, 12020. [Google Scholar] [CrossRef]
- Song, J.Z.; Yip, Y.K.; Han, Q.B.; Qiao, C.F.; Xu, H.X. Rapid Determination of Polyprenylated Xanthones in Gamboge Resin of Garcinia Hanburyi by HPLC. J. Sep. Sci. 2007, 30, 304–309. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Patel, K.; Owaid, I.H.; Singh, M. Tumor Neovasculature-Targeted Cationic PEGylated Liposomes of Gambogic Acid for the Treatment of Triple-Negative Breast Cancer. Drug Deliv. 2016, 23, 1232–1241. [Google Scholar] [CrossRef]
- Saxena, V.; Hussain, M.D. Poloxamer 407/TPGS Mixed Micelles for Delivery of Gambogic Acid to Breast and Multidrug-Resistant Cancer. Int. J. Nanomed. 2012, 7, 713–721. [Google Scholar] [CrossRef]
- Deng, Y.X.; Pan, S.L.; Zhao, S.Y.; Wu, M.Q.; Sun, Z.Q.; Chen, X.H.; Shao, Z.Y. Cytotoxic Alkoxylated Xanthones from the Resin of Garcinia hanburyi. Fitoterapia 2012, 83, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, S.; Li, S.; Zhang, R.; Peng, A.; Chen, L. In Vitro and in Vivo Antiangiogenic Activity of Caged Polyprenylated Xanthones Isolated from Garcinia Hanburyi Hook. F. Molecules 2013, 18, 15305–15313. [Google Scholar] [CrossRef]
- Khaing, E.M.; Phaechamud, T.; Intaraphairot, T. Synergistic Anticancer Activity of Cinnamon Bark Oil and Imatinib Mesylate Combination on Colorectal Cancer Cell Lines. Key Eng. Mater. 2022, 914, 93–98. [Google Scholar] [CrossRef]












| Formula | pH | Viscosity (cP) | Injectability | |
|---|---|---|---|---|
| Force (N) | Work (N·mm) | |||
| NG25 | 7.86 ± 0.06 | 38.10 ± 0.00 | 0.78 ± 0.11 | 2.60 ± 0.17 |
| NG30 | 7.79 ± 0.05 | 43.66 ± 0.00 | 0.90 ± 0.04 | 3.21 ± 0.61 |
| NG40 | 7.58 ± 0.06 | 62.97 ± 0.46 | 1.07 ± 0.14 | 3.13 ± 1.11 |
| NG50 | 7.34 ± 0.05 | ND | 1.07 ± 0.28 | 5.66 ± 0.49 |
| DG25 | 6.86 ± 0.12 | 23.81 ± 0.00 | 0.68 ± 0.15 | 1.53 ± 0.25 |
| DG30 | 6.84 ± 0.03 | 39.69 ± 0.00 | 0.86 ± 0.13 | 2.85 ± 0.36 |
| DG40 | 6.80 ± 0.02 | 132.60 ± 0.00 | 1.10 ± 0.08 | 5.73 ± 0.51 |
| DG50 | 6.25 ± 0.21 | 810.03 ± 3.33 | 1.38 ± 0.07 | 4.55 ± 0.53 |
| Formula | Zero Order | First Order | Higuchi Order | Korsmeyer-Peppas | ||
|---|---|---|---|---|---|---|
| r2 | r2 | r2 | r2 | n | Release Mechanism | |
| 1% IM-NG | 0.269 | 0.600 | 0.756 | 0.951 | 0.234 | quasi-Fickian diffusion |
| 1% IM-DG | 0.509 | 0.672 | 0.889 | 0.906 | 0.402 | quasi-Fickian diffusion |
| 5% IM-NG | 0.341 | 0.721 | 0.862 | 0.890 | 0.246 | quasi-Fickian diffusion |
| 5% IM-DG | 0.454 | 0.903 | 0.924 | 0.961 | 0.366 | quasi-Fickian diffusion |
| Formula | Conc. (% w/w) | |||
|---|---|---|---|---|
| IM | Gamboge | NMP | DMSO | |
| NG25 | 25 | 75 | ||
| NG30 | 30 | 70 | ||
| NG40 | 40 | 60 | ||
| NG50 | 50 | 50 | ||
| DG25 | 25 | 75 | ||
| DG30 | 30 | 70 | ||
| DG40 | 40 | 60 | ||
| DG50 | 50 | 50 | ||
| 1%IM-GN | 1 | 30 | 69 | - |
| 1%IM-GD | 1 | 30 | - | 69 |
| 5%IM-GN | 5 | 30 | 65 | - |
| 5%IM-GD | 5 | 30 | - | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jitrangsri, K.; Khaing, E.M.; Intaraphairot, T.; Phaechamud, T.; Mahadlek, J. Injectable Gamboge-Based In Situ Gel for Sustained Delivery of Imatinib Mesylate. Gels 2023, 9, 737. https://doi.org/10.3390/gels9090737
Jitrangsri K, Khaing EM, Intaraphairot T, Phaechamud T, Mahadlek J. Injectable Gamboge-Based In Situ Gel for Sustained Delivery of Imatinib Mesylate. Gels. 2023; 9(9):737. https://doi.org/10.3390/gels9090737
Chicago/Turabian StyleJitrangsri, Kritamorn, Ei Mon Khaing, Torsak Intaraphairot, Thawatchai Phaechamud, and Jongjan Mahadlek. 2023. "Injectable Gamboge-Based In Situ Gel for Sustained Delivery of Imatinib Mesylate" Gels 9, no. 9: 737. https://doi.org/10.3390/gels9090737