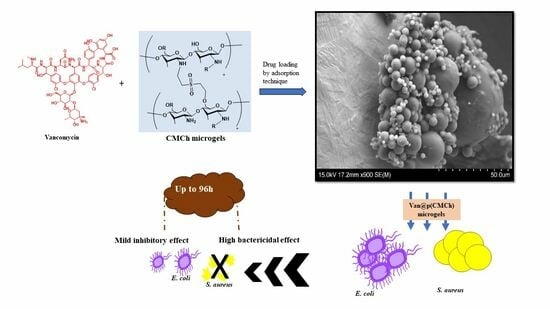
Carboxymethyl Chitosan Microgels for Sustained Delivery of Vancomycin and Long-Lasting Antibacterial Effects
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of CMCh Microgels
4.3. Degradation Profile of CMCh Microgels
4.4. In Vitro Cell Compatibility Studies of CMCh Microgels
4.5. Drug Delivery Abilities of CMCh Microgels
4.6. Antibacterial Activities of Van@CMCh Microgels
4.6.1. Broth Micro-Titer Dilution Assay
4.6.2. Zone of Inhibition Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan Amphiphilic Derivatives. Chemistry and Applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Sommano, S.R.; Rachtanapun, P.; Kantrong, N.; Ruksiriwanich, W.; Kumpugdee-Vollrath, M.; Jantrawut, P. Development of Carboxymethyl Chitosan Nanoparticles Prepared by Ultrasound-Assisted Technique for a Clindamycin HCl Carrier. Polymers 2022, 14, 1736. [Google Scholar] [CrossRef]
- Wu, F.; Meng, G.; He, J.; Wu, Y.; Gu, Z. Antibiotic-Loaded Chitosan Hydrogel with Superior Dual Functions: Antibacterial Efficacy and Osteoblastic Cell Responses. ACS Appl. Mater. Interfaces 2014, 6, 10005–10013. [Google Scholar] [CrossRef]
- Jaikumar, D.; Sajesh, K.; Soumya, S.; Nimal, T.; Chennazhi, K.; Nair, S.V.; Jayakumar, R. Injectable alginate-O-carboxymethyl chitosan/nano fibrin composite hydrogels for adipose tissue engineering. Int. J. Biol. Macromol. 2015, 74, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kzk, A.-A.; Mhs, M. Hematology, bacteriology and antibiotic resistance in milk of water buffalo with subclinical mastitis. OJVRTM Online J. Vet. Res. 2019, 23, 1–8. [Google Scholar]
- Tang, Y.; Sun, J.; Fan, H.; Zhang, X. An improved complex gel of modified gellan gum and carboxymethyl chitosan for chondrocytes encapsulation. Carbohydr. Polym. 2012, 88, 46–53. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, L.; Zhang, X.; Zhao, Y.; Wei, S.; Zhai, M. Radiation synthesis of gelatin/CM-chitosan/β-tricalcium phosphate composite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2012, 32, 994–1000. [Google Scholar] [CrossRef]
- Phan, M.T.T.; Pham, L.N.; Nguyen, L.H.; To, L.P. Investigation on Synthesis of Hydrogel Starting from Vietnamese Pineapple Leaf Waste-Derived Carboxymethylcellulose. J. Anal. Methods Chem. 2021, 2021, 6639964. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Zhao, Y.; Tong, Z.; Chen, S. Superabsorbent Sponge and Membrane Prepared by Polyelectrolyte Complexation of Carboxymethyl Cellulose/Hydroxyethyl Cellulose-Al3+. Bioresources 2015, 10, 6479–6495. [Google Scholar] [CrossRef]
- Das, S.S.; Kar, S.; Singh, S.K.; Hussain, A.; Verma, P.; Beg, S. Carboxymethyl chitosan in advanced drug-delivery applications. In Chitosan in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 323–360. [Google Scholar] [CrossRef]
- Pakzad, Y.; Fathi, M.; Omidi, Y.; Mozafari, M.; Zamanian, A. Synthesis and characterization of timolol maleate-loaded quaternized chitosan-based thermosensitive hydrogel: A transparent topical ocular delivery system for the treatment of glaucoma. Int. J. Biol. Macromol. 2020, 159, 117–128. [Google Scholar] [CrossRef]
- Zhao, D.; Song, H.; Zhou, X.; Chen, Y.; Liu, Q.; Gao, X.; Zhu, X.; Chen, D. Novel facile thermosensitive hydrogel as sustained and controllable gene release vehicle for breast cancer treatment. Eur. J. Pharm. Sci. 2019, 134, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Utami, R.N.; Layadi, P.; Himawan, A.; Juniarti, N.; Anjani, Q.K.; Utomo, E.; Mardikasari, S.A.; Arjuna, A.; Donnelly, R.F. Thermosensitive and mucoadhesive in situ ocular gel for effective local delivery and antifungal activity of itraconazole nanocrystal in the treatment of fungal keratitis. Int. J. Pharm. 2021, 602, 120623. [Google Scholar] [CrossRef]
- Iriventi, P.; Gupta, N.V.; Osmani, R.A.M.; Balamuralidhara, V. Design & development of nanosponge loaded topical gel of curcumin and caffeine mixture for augmented treatment of psoriasis. DARU J. Pharm. Sci. 2020, 28, 489–506. [Google Scholar] [CrossRef]
- Gonçalves, R.C.; Signini, R.; Rosa, L.M.; Dias, Y.S.P.; Vinaud, M.C.; Junior, R.D.S.L. Carboxymethyl chitosan hydrogel formulations enhance the healing process in experimental partial-thickness (second-degree) burn wound healing. Acta Cir. Bras. 2021, 36, e360303. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Rao, K.M.; Sudhakar, K.; Suneetha, M.; Won, S.Y.; Han, S.S. Fungal-derived carboxymethyl chitosan blended with polyvinyl alcohol as membranes for wound dressings. Int. J. Biol. Macromol. 2021, 190, 792–800. [Google Scholar] [CrossRef]
- Pandit, A.H.; Nisar, S.; Imtiyaz, K.; Nadeem, M.; Mazumdar, N.; Alam Rizvi, M.M.; Ahmad, S. Injectable, Self-Healing, and Biocompatible N,O-Carboxymethyl Chitosan/Multialdehyde Guar Gum Hydrogels for Sustained Anticancer Drug Delivery. Biomacromolecules 2021, 22, 3731–3745. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, B.; Jia, Y.; Hou, W.; Su, C. Preparation of Biocompatible Carboxymethyl Chitosan Nanoparticles for Delivery of Antibiotic Drug. BioMed Res. Int. 2013, 2013, 236469. [Google Scholar] [CrossRef]
- Ullah, K.; Sohail, M.; Murtaza, G.; Khan, S.A. Natural and synthetic materials based CMCh/PVA hydrogels for oxaliplatin delivery: Fabrication, characterization, In-Vitro and In-Vivo safety profiling. Int. J. Biol. Macromol. 2018, 122, 538–548. [Google Scholar] [CrossRef]
- Lyu, Y.; Azevedo, H.S. Supramolecular Hydrogels for Protein Delivery in Tissue Engineering. Molecules 2021, 26, 873. [Google Scholar] [CrossRef]
- Lo, W.-H.; Deng, F.-S.; Chang, C.-J.; Lin, C.-H. Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida albicans, Candida tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.R.; More, M.P.; Patil, P.B.; Mujumdar, A.; Naik, J.B. Statistical optimization of voriconazole nanoparticles loaded carboxymethyl chitosan-poloxamer based in situ gel for ocular delivery: In vitro, ex vivo, and toxicity assessment. Drug Deliv. Transl. Res. 2022, 12, 3063–3082. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K.; Thakur, A.; Alexander, A.; Ajazuddin; Badwaik, H.; Tripathi, D.K. Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharm. Sin. B 2012, 2, 439–449. [Google Scholar] [CrossRef]
- Buranachai, T.; Praphairaksit, N.; Muangsin, N. Chitosan/Polyethylene Glycol Beads Crosslinked with Tripolyphosphate and Glutaraldehyde for Gastrointestinal Drug Delivery. AAPS PharmSciTech 2010, 11, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent Advances of Chitosan-Based Injectable Hydrogels for Bone and Dental Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 3063–3082. [Google Scholar] [CrossRef]
- Liu, Q.; Zuo, Q.; Guo, R.; Hong, A.; Li, C.; Zhang, Y.; He, L.; Xue, W. Fabrication and characterization of carboxymethyl chitosan/poly(vinyl alcohol) hydrogels containing alginate microspheres for protein delivery. J. Bioact. Compat. Polym. 2015, 30, 397–411. [Google Scholar] [CrossRef]
- Mathew, S.A.; Arumainathan, S. Crosslinked Chitosan–Gelatin Biocompatible Nanocomposite as a Neuro Drug Carrier. ACS Omega 2022, 7, 18732–18744. [Google Scholar] [CrossRef] [PubMed]
- Quadrado, R.F.N.; Fajardo, A.R. Microparticles based on carboxymethyl starch/chitosan polyelectrolyte complex as vehicles for drug delivery systems. Arab. J. Chem. 2020, 13, 2183–2194. [Google Scholar] [CrossRef]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved Glycopeptide Antibacterial Drugs: Mechanism of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Preuss, C.V.; Bernice, F. Vancomycin; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gomceli, U.; Vangala, S.; Zeana, C.; Kelly, P.J.; Singh, M. An Unusual Case of Ototoxicity with Use of Oral Vancomycin. Case Rep. Infect. Dis. 2018, 2018, 2980913. [Google Scholar] [CrossRef] [PubMed]
- Beumier, M.; Roberts, J.A.; Kabtouri, H.; Hites, M.; Cotton, F.; Wolff, F.; Lipman, J.; Jacobs, F.; Vincent, J.-L.; Taccone, F.S. A new regimen for continuous infusion of vancomycin during continuous renal replacement therapy. J. Antimicrob. Chemother. 2013, 68, 2859–2865. [Google Scholar] [CrossRef]
- Peng, Y.M.; Li, C.-Y.M.; Yang, Z.-L.M.; Shi, W.M. Adverse reactions of vancomycin in humans. Medicine 2020, 99, e22376. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Cao, Y.; Kumeria, T.; Blaskovich, M.A.T.; Falconer, J.R.; Popat, A. Engineering mesoporous silica nanoparticles towards oral delivery of vancomycin. J. Mater. Chem. B 2021, 9, 7145–7166. [Google Scholar] [CrossRef]
- Li, D.; Tang, G.; Yao, H.; Zhu, Y.; Shi, C.; Fu, Q.; Yang, F.; Wang, X. Formulation of pH-responsive PEGylated nanoparticles with high drug loading capacity and programmable drug release for enhanced antibacterial activity. Bioact. Mater. 2022, 16, 47–56. [Google Scholar] [CrossRef]
- Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef]
- Cauda, V.; Onida, B.; Platschek, B.; Mühlstein, L.; Bein, T. Large antibiotic molecule diffusion in confined mesoporous silica with controlled morphology. J. Mater. Chem. 2008, 18, 5888–5899. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Ganji, F. Vancomycin-loaded HPMC microparticles embedded within injectable thermosensitive chitosan hydrogels. Prog. Biomater. 2017, 6, 49–56. [Google Scholar] [CrossRef]
- Le Ray, A.-M.; Chiffoleau, S.; Iooss, P.; Grimandi, G.; Gouyette, A.; Daculsi, G.; Merle, C. Vancomycin encapsulation in biodegradable poly(ε-caprolactone) microparticles for bone implantation. Influence of the formulation process on size, drug loading, in vitro release and cytocompatibility. Biomaterials 2003, 24, 443–449. [Google Scholar] [CrossRef]
- Li, S.; Shi, X.; Xu, B.; Wang, J.; Li, P.; Wang, X.; Lou, J.; Li, Z.; Yang, C.; Li, S.; et al. In vitro drug release and antibacterial activity evaluation of silk fibroin coated vancomycin hydrochloride loaded poly (lactic-co-glycolic acid) (PLGA) sustained release microspheres. J. Biomater. Appl. 2022, 36, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Pan, Q.; Zheng, Z.; Chen, Y.; Chen, Y.; Weng, S.; Huang, L. pH-responsive and porous vancomycin-loaded PLGA microspheres: Evidence of controlled and sustained release for localized inflammation inhibition in vitro. RSC Adv. 2018, 8, 37424–37432. [Google Scholar] [CrossRef]
- Lai, J.-Y. Relationship between structure and cytocompatibility of divinyl sulfone cross-linked hyaluronic acid. Carbohydr. Polym. 2014, 101, 203–212. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S.; Sahiner, M.; Al-Lohedan, H. The synthesis of desired functional groups on PEI microgel particles for biomedical and environmental applications. Appl. Surf. Sci. 2015, 354, 380–387. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Investigation of the swelling behavior of crosslinked hyaluronic acid films and hydrogels produced using homogeneous reactions. J. Appl. Polym. Sci. 2008, 109, 923–931. [Google Scholar] [CrossRef]
- Patricia, J.J.; Dhamoon, A.S. Physiology, Digestion; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Akiba, Y.; Mizumori, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am. J. Physiol. Liver Physiol. 2007, 293, G1223–G1233. [Google Scholar] [CrossRef]
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic pH and High-H2O2 Dual Tumor Microenvironment-Responsive Nanocatalytic Graphene Oxide for Cancer Selective Therapy and Recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166. [Google Scholar] [CrossRef]
- Pugliese, S.C.; Poth, J.M.; Fini, M.A.; Olschewski, A.; El Kasmi, K.C.; Stenmark, K.R. The role of inflammation in hypoxic pulmonary hypertension: From cellular mechanisms to clinical phenotypes. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L229–L252. [Google Scholar] [CrossRef]
- Xiao, K.; Lin, T.-Y.; Lam, K.S.; Li, Y. A facile strategy for fine-tuning the stability and drug release of stimuli-responsive cross-linked micellar nanoparticles towards precision drug delivery. Nanoscale 2017, 9, 7765–7770. [Google Scholar] [CrossRef]
- Foster, G.A.; Headen, D.M.; González-García, C.; Salmerón-Sánchez, M.; Shirwan, H.; García, A.J. Protease-degradable microgels for protein delivery for vascularization. Biomaterials 2017, 113, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Kanno, M.; Miharada, K.; Ogawa, S.; Hiroyama, T.; Saijo, K.; Nakamura, Y. Mesenchymal Progenitors Able to Differentiate into Osteogenic, Chondrogenic, and/or Adipogenic Cells In Vitro Are Present in Most Primary Fibroblast-Like Cell Populations. Stem Cells 2007, 25, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Wadajkar, A.S.; Ahn, C.; Nguyen, K.T.; Zhu, Q.; Komabayashi, T. In Vitro Cytotoxicity Evaluation of Four Vital Pulp Therapy Materials on L929 Fibroblasts. ISRN Dent. 2014, 2014, 191068. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; Salamat, M.K.F.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017, 6, 47. [Google Scholar] [CrossRef]
- Sahiner, M.; Demirci, S.; Sahiner, N. Enhanced Bioactive Properties of Halloysite Nanotubes via Polydopamine Coating. Polymers 2022, 14, 4346. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- ElShaer, A.; Ghatora, B.; Mustafa, S.; Alany, R.G. Contact lenses as drug reservoirs & delivery systems: The successes & challenges. Ther. Deliv. 2014, 5, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Bărăian, A.-I.; Iacob, B.-C.; Sorițău, O.; Tomuță, I.; Tefas, L.R.; Barbu-Tudoran, L.; Șușman, S.; Bodoki, E. Ruxolitinib-Loaded Imprinted Polymeric Drug Reservoir for the Local Management of Post-Surgical Residual Glioblastoma Cells. Polymers 2023, 15, 965. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Yilmaz, A.S.; Demirci, S.; Sahiner, N. Physically and Chemically Crosslinked, Tannic Acid Embedded Linear PEI-Based Hydrogels and Cryogels with Natural Antibacterial and Antioxidant Properties. Biomedicines 2023, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Kralik, P.; Beran, V.; Pavlik, I. Enumeration of Mycobacterium avium subsp. paratuberculosis by quantitative real-time PCR, culture on solid media and optical densitometry. BMC Res. Notes 2012, 5, 114. [Google Scholar] [CrossRef]







| Kinetic Model | Parameters | Van@CMCh Microgels |
|---|---|---|
| Zero order | k0 | 25.582 |
| R2 | 0.9652 | |
| First order | k1 | 0.327 |
| R2 | 0.9264 | |
| Higuchi | kH | 30.667 |
| R2 | 0.8257 | |
| Korsmeyer–Peppas | kKP | 22.437 |
| n | 1.245 | |
| R2 | 0.9763 |
| Microorganisms | E. coli | S. aureus | ||
|---|---|---|---|---|
| Sample | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) |
| CMCh | - | - | - | - |
| CMCh microgels | - | - | - | - |
| Van@CMCh microgels | 64 | - | 8 | 8 |
| Vancomycin * | 15 | 250 | 0.2 | 0.2 |
| Inhibition Zone Diameter (mm) | 24 h | 48 h | 96 h |
|---|---|---|---|
| E. coli | - | - | 11.5 ± 1 |
| S. aureus | 11.6 ± 1.5 | 12.5 ± 1 | 14.5 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahiner, M.; Yilmaz, A.S.; Ayyala, R.S.; Sahiner, N. Carboxymethyl Chitosan Microgels for Sustained Delivery of Vancomycin and Long-Lasting Antibacterial Effects. Gels 2023, 9, 708. https://doi.org/10.3390/gels9090708
Sahiner M, Yilmaz AS, Ayyala RS, Sahiner N. Carboxymethyl Chitosan Microgels for Sustained Delivery of Vancomycin and Long-Lasting Antibacterial Effects. Gels. 2023; 9(9):708. https://doi.org/10.3390/gels9090708
Chicago/Turabian StyleSahiner, Mehtap, Aynur S. Yilmaz, Ramesh S. Ayyala, and Nurettin Sahiner. 2023. "Carboxymethyl Chitosan Microgels for Sustained Delivery of Vancomycin and Long-Lasting Antibacterial Effects" Gels 9, no. 9: 708. https://doi.org/10.3390/gels9090708