Effective Removal of Cu2+ Ions from Aqueous Media Using Poly(acrylamide-co-itaconic acid) Hydrogels in a Semi-Continuous Process
Abstract
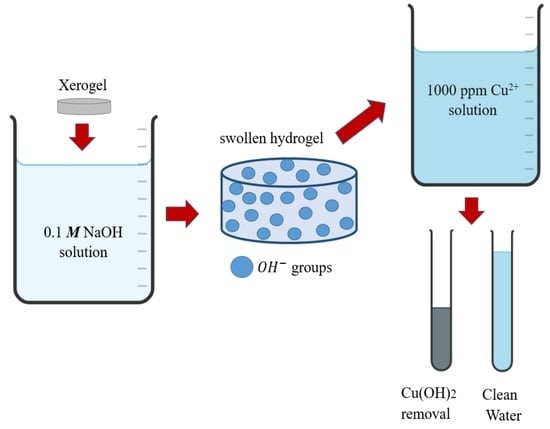
:1. Introduction
2. Results
2.1. Conversion
2.2. Metal Ion Recovery
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Hydrogel Synthesis Reactions
4.3. Conversion Determination
4.4. Batch Study of Removal of Cu2+ Ions in Aqueous Solution
4.5. Semi-Continuous Study of Removal of Cu2+ Ions in Aqueous Solution
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, L.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef]
- Araya, M.; Chen, B.; Klevay, L.M.; Strain, J.J.; Johnson, L.; Robson, P.; Shi, W.; Nielsen, F.; Zhu, H.; Olivares, M.; et al. Confirmation of an acute no-observed-adverse-effect and low-observed-adverse-effect level for copper in bottled drinking water in a multi-site international study. Regul. Toxicol. Pharmacol. 2003, 38, 389–399. [Google Scholar] [CrossRef]
- Committee on Copper in Drinking Water and National Research Council. Copper in Drinking Water; National Academies Press (US): Washington, DC, USA, 2000.
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Lipowsky, H.; Arpaci, E. Copper in the Automotive Industry, 1st ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 3–9. [Google Scholar]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Ku, Y.; Jung, I.L. Photocatalytic reduction of Cr(VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res. 2001, 35, 135–142. [Google Scholar] [CrossRef]
- Basha, C.A.; Bhadrinarayana, N.S.; Anantharaman, N.; Begum, K.M.M.S. Heavy metal removal from copper smelting effluent using electrochemical cylindrical flow reactor. J. Hazard. Mater. 2008, 152, 71–78. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Salmani, N.; Heidari, A.; Rezaei, M.R. Bio-synthesis of palladium nanoparticle using Spirulina platensis alga extract and its application as adsorbent. Surf. Interfaces. 2018, 10, 136–143. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Shapiro, J.M.; Oyen, M.L. Hydrogel composite materials for tissue engineering scaffolds. JOM 2013, 65, 505–516. [Google Scholar] [CrossRef]
- Daniele, M.A.; Adams, A.A.; Naciri, J.; North, S.H.; Ligler, F.S. Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials 2014, 35, 1845–1856. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Chen, G.; Tang, W.; Wang, X.; Zhao, X.; Chen, C.; Zhu, Z. Applications of Hydrogels with Special Physical Properties in Biomedicine. Polymers 2019, 11, 1420–1437. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Ghobashy, M.M. The application of natural polymer-based hydrogels for agriculture. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–356. [Google Scholar]
- Wang, W.B.; Huang, D.J.; Kang, Y.R.; Wang, A.Q. One-step in situ fabrication of a granular semi-IPN hydrogel based on chitosan and gelatin for fast and efficient adsorption of Cu2+ ion. Colloids Surf. B Biointerfaces 2013, 106, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, Y.; Wang, F.; Wang, A. Monolithic supermacroporous hydrogel prepared from high internal phase emulsions (HIPEs) for fast removal of Cu2+ and Pb2+. Chem. Eng. J. 2016, 284, 422–430. [Google Scholar] [CrossRef]
- Abdelwahab, H.E.; Hassan, S.Y.; Mostafa, M.A.; El Sadek, M.M. Synthesis and characterization of glutamic-chitosan hydrogel for copper and nickel removal from wastewater. Molecules 2016, 21, 684–698. [Google Scholar] [CrossRef]
- Firdaus, V.; Idris, M.S.F.; Yusoff, S.F.M. Adsorption of Nickel Ion in Aqueous Using Rubber-Based Hydrogel. J. Polym. Environ. 2019, 27, 1770–1780. [Google Scholar] [CrossRef]
- Sahraei, R.; Ghaemy, M. Synthesis of modified gum tragacanth/graphene oxide composite hydrogel for heavy metal ions removal and preparation of silver nanocomposite for antibacterial activity. Carbohydr. Polym. 2017, 157, 823–833. [Google Scholar] [CrossRef]
- Ramos, M.L.P.; González, J.A.; Albornoz, S.G.; Pérez, C.J.; Villanueva, M.E.; Giorgieri, S.A.; Copello, G.J. Chitin hydrogel reinforced with TiO2 nanoparticles as an arsenic sorbent. Chem. Eng. J. 2016, 285, 581–587. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Ma, J.; Tang, Y.; Zeng, Z.; Luo, S. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res. 2016, 89, 151–160. [Google Scholar] [CrossRef]
- Wu, B.; Yan, D.Y.S.; Khan, M.; Zhang, Z.; Lo, I.M.C. Application of Magnetic Hydrogel for Anionic Pollutants Removal from Wastewater with Adsorbent Regeneration and Reuse. J. Hazard. Toxic Radioact. Waste. 2017, 21, 04016008. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef]
- Serag, E.; El Nemr, A.; El-Maghraby, A. Synthesis of highly effective novel graphene oxide-polyethylene glycol-polyvinyl alcohol nanocomposite hydrogel for copper removal. J. Water Environ. Nanotechnol. 2017, 2, 223–234. [Google Scholar] [CrossRef]
- Vesali-Naseh, M.; Barati, A.; Vesali Naseh, M.R. Efficient copper removal from wastewater through montmorillonite-supported hydrogel adsorbent. Water Environ. Res. 2019, 91, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, R.; Chen, M.; Li, Z.; Qin, T.; Qian, Y.; Zhao, S.; Liu, M.; Zeng, Q.; Shen, J. Removal of copper ions from water using polysaccharide-constructed hydrogels. Carbohydr. Polym. 2019, 209, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, S.; Cui, S.; Tang, Y.; Pei, Z.; Duan, H. Magnetic-controlled aerogels from carboxylated cellulose and MnFe 2 O 4 as a novel adsorbent for removal of Cu (II). Cellulose 2019, 26, 5051–5063. [Google Scholar]
- Shen, Y.; Wang, Q.; Wang, Y.; He, Y.F.; Song, P.; Wang, R.M. Itaconic copolymer modified loess for high-efficiently removing copper ions from wastewater. J. Dispers. Sci. Technol. 2019, 40, 794–801. [Google Scholar] [CrossRef]
- Olvera-Sosa, M.; Guerra-Contreras, A.; Gómez-Durán, C.F.; González-García, R.; Palestino, G. Tuning the pH-responsiveness capability of poly (acrylic acid-co-itaconic acid)/NaOH hydrogel: Design, swelling, and rust removal evaluation. J. Appl. Polym. Sci. 2020, 137, 48403–48416. [Google Scholar] [CrossRef]
- Nie, L.; Chang, P.; Liang, S.; Hu, K.; Hua, D.; Liu, S.; Sun, J.; Sun, M.; Wang, T.; Okoro, O.V.; et al. Polyphenol rich green tea waste hydrogel for removal of copper and chromium ions from aqueous solution. Clean. Eng. Technol. 2021, 4, 100167. [Google Scholar]
- Wang, H.; Fang, S.; Zuo, M.; Li, Z.; Yu, X.; Tang, X.; Sun, Y.; Yang, S.; Zeng, X.; Lin, L. Removal of copper ions by cellulose nanocrystal-based hydrogel and reduced adsorbents for its catalytic properties. Cellulose 2022, 29, 4525–4537. [Google Scholar] [CrossRef]
- Lin, Z.; Li, F.; Liu, X.; Su, J. Preparation of corn starch/acrylic acid/itaconic acid ion exchange hidrogel and its adsorption properties for copper and lead ions in wastewater. Colloids Surf. A Physicochem. 2023, 671, 131668–131679. [Google Scholar] [CrossRef]
- Hernández, J.A.; Zárate-Navarro, M.A.; Alvarado, A.G. Study and comparison of several methods to remove Ni(II) ions in aqueous solutions using poly(acrylamide-co-itaconic acid) hydrogels. J. Polym. Res. 2020, 27, 238–245. [Google Scholar] [CrossRef]








| Experiment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| massCuCl2 (g) | 18 | 16 | 14 | 12 | 10 | 8 | 6 | 4 | 2 |
| massNaOH (g) | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 |
| molOH−/molCu2+ | 0.71 | 1.59 | 2.74 | 4.27 | 6.37 | 9.52 | 14.78 | 25.37 | 56.36 |
| RCu2+(%) | 30 | 41 | 98 | 99 | 98 | 99 | 95 | 93 | 98 |
| [NaOH] M | W (%) | mgCu2+/gxerogel | RCu2+ (%) |
|---|---|---|---|
| 0.1 | 64 | 183 | 98.52 |
| 0.2 | 55 | 194 | 98.44 |
| 0.3 | 53 | 205 | 99.10 |
| 0.4 | 50 | 211 | 99.68 |
| Adsorbents Materials | Qmax (mg/g) | Reference |
|---|---|---|
| Graphene oxide-polyethylene glycol and polyvinyl alcohol (GO-PEG-PVA) triple network hydrogel | 917 | [33] |
| Hybrid hydrogel of acrylic acid monomer/wheat bran/montmorillonite | 17.64 | [34] |
| Hydrogels comprised of polysaccharide salecan injerted with poly(3-sulfopropyl methacrylate potassium salt). | 107.2 | [35] |
| Aerogels comprised of carboxylated cellulose and MnFe2O4. | 73.70 | [36] |
| Hydrogels comprised of Loess of clay/Itaconic acid/2-Hydroxyethyl methacrylate/N-vinyl-2-pyrrolidone | 594.43 | [37] |
| Poly(acrylic acid-co-itaconic acid)/NaOH hydrogel | 85 | [38] |
| Polyvinyl alcohol/alginate/iron oxide nanoparticles (PAI) hydrogels | 60 | [39] |
| Carboxymethylcellulose sodium/polyvinyl alcohol (PVA)/Cellulose nanocrystals hydrogels | 108.8 | [40] |
| Corn starch/acrylic acid/itaconic acid ion exchange hydrogel | 699.31 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortes Ortega, J.A.; Hernández-Montelongo, J.; Hernández-Montelongo, R.; Alvarado Mendoza, A.G. Effective Removal of Cu2+ Ions from Aqueous Media Using Poly(acrylamide-co-itaconic acid) Hydrogels in a Semi-Continuous Process. Gels 2023, 9, 702. https://doi.org/10.3390/gels9090702
Cortes Ortega JA, Hernández-Montelongo J, Hernández-Montelongo R, Alvarado Mendoza AG. Effective Removal of Cu2+ Ions from Aqueous Media Using Poly(acrylamide-co-itaconic acid) Hydrogels in a Semi-Continuous Process. Gels. 2023; 9(9):702. https://doi.org/10.3390/gels9090702
Chicago/Turabian StyleCortes Ortega, Jorge Alberto, Jacobo Hernández-Montelongo, Rosaura Hernández-Montelongo, and Abraham Gabriel Alvarado Mendoza. 2023. "Effective Removal of Cu2+ Ions from Aqueous Media Using Poly(acrylamide-co-itaconic acid) Hydrogels in a Semi-Continuous Process" Gels 9, no. 9: 702. https://doi.org/10.3390/gels9090702