1. Introduction
Overweight and obesity are among the most prevalent risk factors associated with a number of acute and chronic health problems including type II diabetes, hypertension, osteoarthritis, cancer, and cardiovascular disease. Obesity in turn is linked to various causes, including a person’s genotype, a sedentary lifestyle, and excessive food consumption [
1,
2]. At present, a few drugs are available in the market, including sibutramine and rimonabant, that function as appetite suppressants to reduce obesity. Other therapeutics such as orlistat are designed to prevent fat absorption. However, most products display limited efficacy, along with major adverse effects affecting poor tolerability and safety [
3]. In 2010 A.D., the United States Food and Drug Administration withdrew sibutramine and fenfluramine. Hence, considerable effort has been directed toward finding alternative and improved therapeutics for reducing obesity, with the investigation of herbal medicines receiving significant attention [
4].
Garcinia atroviridis Griff. Ex T. Anderson also known as garcinia, Malabar tamarind, belongs to the plant family of
Clusiaceae and is distributed mainly in Southeast Asia [
5]. The native name in Thailand is “Som-Khaek” [
6,
7]. The dried fruit rind of garcinia has been used extensively for centuries as a condiment and flavoring agent in culinary dishes. The leaves and fruit have also been exploited in traditional medicine to exploit their thermogenic properties and increase metabolic activity and aid digestion. The herbal preparations or natural medicines prepared from garcinia rinds have also been applied in the treatment of inflammatory ailments and bowel conditions [
8,
9,
10].
Garcinia contains various phytochemicals including flavonoids and organic acids, among which hydroxycitric acid (HCA, C
6H
8O
8, molecular weight 208.12 g/mol) (
Figure 1) has been identified as an anti-obesity agent and a potential supplement for weight management [
8]. HCA is water soluble and stable at room temperature in closed containers but incompatible with oxidizing agents. The
Garcinia atroviridis extracts for commercial products are isolated from fruit rind and contain about 50% HCA.
Numerous studies have shown that consumption of HCA suppresses appetite and lowers the production of cholesterol and fatty acids. HCA reduces appetite, lipogenesis, and body weight by inhibiting an enzyme involved in fat storage in adipose tissue. HCA has been shown to be a competitive inhibitor of ATP citrate lyase [
3,
11,
12]. Moreover, HCA increases hepatic glycogen synthesis, which helps produce a longer-lasting neurosignal from the liver to the brain, indicating satiety and thus prolonging appetite suppression [
8,
10].
HCA possesses significant advantages as a weight loss supplement due to its natural origin and safety profile. Studies both in human [
13] and animal subjects [
14] showed no serious side effects at a high dose of 5000 mg/kg, equivalent to 350 g/kg in humans [
15]. Garcinia extract products currently available in the market are manufactured as conventional oral liquid, tablet, and capsule dosage forms, designed to be taken three times a day to deliver a dose of 1500–2800 mg [
13,
16,
17]. However, the conventional oral dosage forms can result in poor bioavailability due to incomplete drug absorption, degradation in the gastrointestinal tract, and fast emptying times, resulting in poor compliance and inconsistent therapeutic efficacy.
Gastroretentive drug delivery systems (GRDDS) are designed to remain in the stomach for extended time periods following oral administration and gradually release active compounds in a controlled manner [
18]. The reduced frequency of administration is intended to decrease drug dose and improve patient compliance. Various approaches have been investigated to prolong gastric residence time including density variation, the use of bioadhesive systems, and expandable and raft-like delivery devices that float on the stomach contents [
19,
20]. Oral in situ gelling systems have recently been investigated as a novel form of GRDDS. The formulations are administered as a low-viscosity liquid, and gel on exposure to stomach contents to prolong drug release [
21,
22]. The transition from liquid to gel may be induced by a shift in temperature in the case of thermo-gelling xyloglucan [
23] or by the presence of cations in the case of gellan gum [
24], sodium alginate and pectin hydrogels [
22,
23,
25].
The purpose of this study was to develop an oral, liquid gastroretentive, in situ gelling formulation incorporating garcinia extract for sustained drug release in the stomach. Moreover, gelation of the dosage form and expansion on contact with gastric fluid was expected to augment the appetite suppression action of HCA. The anti-obesity activity of the formulation was assessed in vitro using a mouse 3T3-L1 cell line and the anti-inflammatory properties were investigated by using RAW 264.7 macrophage cells.
4. Materials and Methods
4.1. Materials
Garcinia extract powder (~54.6% hydroxycitric acid) was provided by Tha Phra Chan Herb Co., Ltd. (Bangkok, Thailand). HCA standard compound (purity > 93.7%) (MW 208.12 g/mol) was obtained from ChromaDex® Corp. (Los Angeles, CA, USA). Sodium alginate medium viscosity (viscosity of 2% w/w solution ~2000 cps; 25 °C) was purchased from High Science Ltd. (Songkhla, Thailand). Calcium carbonate was obtained from RCI Labscan (Bangkok, Thailand). Hydroxypropyl methyl cellulose (HPMC) Methocel K100LV was a gift from Colorcon Asia Pacific Pte Ltd. (Singapore) and sodium citrate was purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). All other reagents were of analytical grade.
3T3-L1 cells (CL-173TM, fibroblast cell line) and RAW 264.7 cells (TIB-71TM, murine macrophage cell line) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). High glucose Dulbecco’s modified eagle medium (DMEM), Roswell Park Memorial Institute 1640 medium (RPMI-1640), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), penicillin–streptomycin, phosphate buffer saline (PBS; pH 7.4), trypsin EDTA 0.25%, and trypan blue solution were supplied by Gibco® (Invitrogen, CA, USA). Fetal bovine serum (FBS) was purchased from HycloneTM (Cytiva, Bangkok, Thailand). Dimethylsulfoxide (DMSO) was obtained from Amresco® (Solon, OH, USA). Lipopolysaccharide (LPS, from Escherichia coli). Isopropanol was sourced from Fisher Scientific International, Inc. (Hampton, VA, USA). Formalin was a gift from Songklanagarind Hospital (Songkhla, Thailand). Dexamethasone (DEX), 3-isobutyl-1-methylxanthanine (IBMX), insulin, indomethacin, Oil Red O solution 0.5% in isopropanol, and Griess reagent were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of analytical or pharmaceutical grades.
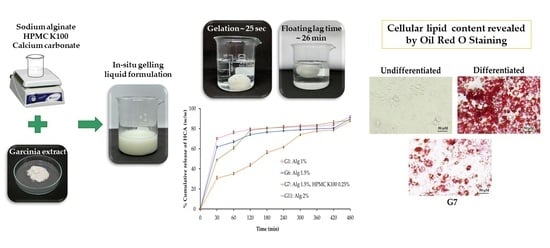
4.2. Preparation of In Situ Gelling Liquid Formulations Loaded with Garcinia Extract
In situ gelling liquid formulations were prepared by combining sodium alginate as a gelling polymer and hydroxypropyl methylcellulose (HPMC) to achieve extended drug release behavior. The composition of the formulation is shown in
Table 6. Briefly, sodium alginate was dissolved in 75 mL deionized water to produce 1, 1.5, and 2%
w/
v solutions, respectively. Sodium citrate (750 mg) was added, and the mixture was stirred until completely dissolved. HPMC K100 was added (except formulations G1, G6, and G11) to the alginate solution to obtain concentrations of 0.25, 0.5, 0.75, or 1%
w/
v, respectively, and stirring was continued to obtain clear solutions. Finally, calcium carbonate as a cross-linking agent (1 g) and garcinia extract (2 g) were added to each sodium alginate/HPMC blended solution, respectively. The liquid formulations were adjusted to 100 mL with deionized water and stored in sealed, light-resistant glass until further use.
4.3. Physicochemical Characterization of In Situ Gelling Liquid Formulations
4.3.1. Physical Appearance and Measurement of pH
The physical appearance of garcinia extract liquid formulations was determined by visual observation of color, and homogeneity. Measurements of pH were obtained using a calibrated digital pH meter (FiveEasy F20, Mettler-Toledo GmBH, Zurich, Switzerland) at room temperature (25 ± 1 °C) in triplicate.
4.3.2. Floating Behavior
The floating behavior of the liquid formulations was investigated by adding 30 mL to 900 mL of 0.1 N hydrochloric acid (pH 1.2) at 37 ± 0.5 °C. The time taken for the formed gel to float to the surface of the 0.1 N hydrochloric acid (pH 1.2) medium was designated “floating lag time” (FLT), while the duration of floating was recorded over 24 h. Each formulation was tested in triplicate.
4.3.3. Gel Density
The density of gels formed by exposure of the liquid formulations to acidic medium was measured as follows. A measuring cylinder containing 75 mL of acidic medium (0.1 N hydrochloric acid; pH 1.2) was weighed (W
1). Liquid formulation (5 mL) was gently added to the measuring cylinder to form a gel and the weight of the cylinder (g) was measured after 30 min (W
2). The volume (mL) of the gel was recorded from the measuring cylinder scale (V). Gel density was calculated using equation [
33]:
4.3.4. Viscosity of In Situ Gelling Liquid Formulations
The viscosity of in situ gelling liquid formulations was measured using a Brookfield digital viscometer (DV-III ultra, Middleboro, MA, USA) with spindle no.64 (LV4). The viscosity of the liquid formulations was measured under different angular velocities (5–70 rpm) at 25 ± 1 °C [
36,
37]. Rheograms were generated for each liquid formulation by plotting viscosity (mPas) versus shear rate (1/S). Each sample was measured in triplicate.
4.3.5. Gel Strength
The gel strength resulting from in situ gelation of liquid formulations was evaluated by modifying the method of Nairy et al. [
36]. A texture analyzer (TA. XT plus Texture Analyzer, Stable Micro Systems, Haslemere, UK) fitted with a 5 kg load cell was employed, and data were analyzed using Exponent software. Gel samples were placed in a Petrie dish and compressed using a 25 mm diameter cylindrical aluminum probe until the gel fractured. The compression speed was set at 1 mm/sec, over a distance of 8 mm, with an acquisition rate of 500 points per second, and a trigger force of 5 g was applied. The load–displacement graph was recorded and the maximum load (g) at gel rupture was reported as the gel strength. Each formulation was measured in triplicate and data were reported as mean ± S.D. (
n = 3).
4.3.6. Volume of the Formed Gel
The volume of the gel formed by in situ gelation of liquid formulations was evaluated using a measuring cylinder. Briefly, 30 mL of liquid formulation (equivalent to one dose) was poured carefully into 200 mL of medium (0.1 N hydrochloric acid; pH 1.2) contained in a 250 mL measuring cylinder. After that, the volume rising was observed on the scale bar of measuring cylinder and recorded. Each formulation was measured in triplicate.
4.3.7. Determination of HCA Content of Liquid Formulations
The HCA loading of the liquid formulations (5 mL samples) was determined by extraction from deionized water (50 mL) contained in a volumetric flask. The mixture was sonicated for 30 min in an ultrasonic water bath (Crest Ultrasonic Corp., Ewing Township, NJ, USA), filtrated by using a 0.45 µm membrane filter (VertiPure™ PVDF(HL), Vertical Chromatography Co., Ltd., Bangkok, Thailand) and diluted to various concentrations in deionized water. The amount of HCA was analyzed by UV–visible spectrophotometry (UV-1900i, SHIMADZU Corporation, Kyoto, Japan) at 210 nm wavelength. The entrapment efficiency (EE) of HCA was calculated according to the following equation:
where A is the measured amount of HCA in the formulation and B is the amount of HCA initially added to the formulation. Data were presented as the mean ± S.D. (
n = 3).
4.3.8. In Vitro Release of HCA from In Situ Gelling Liquid Formulations
Release of HCA from in situ gelling liquid formulations was evaluated using a USP Dissolution Apparatus II (paddle type) (PTWS 120D, Pharma Test Apparatebau AG, Hainburg, Germany). The release medium of 900 mL (0.1 N hydrochloric acid; pH 1.2) was maintained at 37 ± 0.5 °C. A paddle rotation speed of 50 rpm was applied in order to mimic the movement of stomach content. Liquid formulation (30 mL) was injected into the release medium using a disposable syringe and 5 mL was collected at predetermined time intervals (30, 60, 120, 180, 240, 300, 360, 420, and 480 min) and replaced by an equal volume of pre-warmed fresh medium. The release sample was filtered through a 0.45 µm membrane filter (VertiPure™ PVDF(HL)) and the concentration of HCA was measured by UV–visible spectrophotometry (UV-1900i, SHIMADZU Corporation, Kyoto, Japan) at 210 nm absorbance wavelength. The release profiles of HCA were plotted as percent cumulative release (%w/w) versus time (min). Each formulation was tested in triplicate and reported as mean ± S.D.
4.4. Release Kinetics
The release kinetics of HCA from the formulations were investigated by examining the data fit several mathematical models, namely zero-order, first-order, Higuchi model, Korsmeyer–Peppas model, Hixson–Crowell, and Weibull model, using the DDSolver program [
38]. A coefficient of determination (R
2) closest to 1 was considered to indicate the best fit of release data to a particular kinetic model.
4.5. Stability Studies
Stability testing of in situ gelling liquid formulations was performed according to the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH guidelines 2003) on the topic of Q1A (R2): stability testing of new drug substances and products. The physical and chemical stability of selected liquid formulations were tested at intermediate conditions (30 ± 2 °C, 65% ± 5% relative humidity; RH) and at accelerated conditions (45 ± 2 °C, 75% ± 5% RH) [
32]. The optimized formulations were placed in tightly sealed glass containers and stored in a constant climate chamber (Memmert
® HPP260, New York, USA). Meanwhile, the stability of formulations was also studied at 4 ± 2 °C in a refrigerator. After 1, 3, and 6 months, the formulations were evaluated in terms of pH, gelation, floating behavior, density, viscosity, gel strength, and HCA content.
4.6. Biological Assay of In Situ Gelling Liquid Formulations of Garcinia Extract
4.6.1. Cell Culture
Mouse 3T3-L1 preadipocytes were grown in DMEM, supplemented with 10% FBS and 1% penicillin–streptomycin at 37 °C under a humidified 5% CO2 atmosphere. The culture medium was changed every two days and cells were subcultured upon reaching 70% confluence. Murine macrophages (RAW 264.7 cells) were cultured in RPMI 1640 medium, supplemented with 10% FBS and 1% penicillin–streptomycin at 37 °C under a humidified 5% CO2 atmosphere. The cells were subcultured after reaching 100% confluence.
4.6.2. Cell Differentiation
The 3T3-L1 fibroblasts were induced to differentiate into mature adipocytes by exposure to differentiation activation medium (D/A) as previously described [
39]. In brief, differentiation was started by incubation of two days post-confluent cells with D/A media comprising 0.5 mM IBMX, 1 μM DEX, and 10 μg/mL insulin (defined as Day 0). On Day 2, the D/A medium was switched to the maintain medium (D/M, 10 μg/mL insulin in DMEM, high glucose) and refreshed every 2 days until cells were fully differentiated into adipocytes (Day 10–12).
4.6.3. Cell Viability following Exposure to In Situ Gelling Liquid Formulations
The viability of both 3T3-L1 adipocytes [
34,
39,
40] and RAW 264.7 cells [
41] was measured by MTT reduction assay. The 3T3-L1 fibroblasts were seeded into 96-well plates at a density of 5000 cells/well and induced to fully differentiate as described above. The RAW 264.7 cells were seeded at a density of 50,000 cells/well in 96-well plates and grown overnight. 3T3-L1 adipocyte or RAW 264.7 cells were treated with various concentrations of standard, garcinia extract, optimized in situ gelling liquid formulation, and blank formulation for 24 h. The culture medium was removed, and the cells were incubated with 0.5 mg/mL MTT solution at 37 °C for 3 h. The supernatant was removed, and DMSO was added to dissolve the formazan crystals. The absorbance was measured at 570 nm using the microplate reader (Biotek model Power Wave X, Santa Clara, CA, USA), and cell viability was expressed as a percentage of the control value using the following equation:
4.6.4. Assay of Anti-Obesity Activity
The inhibitory effect of standard, garcinia extract, optimized in situ gelling liquid formulation and blank formulation on lipid accumulation in 3T3-L1 cells was examined using Oil Red O staining. In brief, 3T3-L1 preadipocytes were seeded in 48-well plates at a density of 20,000 cells/well and induced to differentiate for 10 days in the absence or presence of standard, garcinia extract, optimized formulation, and blank formulation. Cells were washed with PBS and fixed with 10% formaldehyde for 60 min. The fixed cells were subsequently washed twice with 60% isopropanol and maintained at room temperature (RT) to dry completely. Oil Red O solution (200 µL) was added to each well plate and incubated at RT for 15 min. The cells were washed 4–5 times with deionized water to remove excess dye, prior to visualization of lipid staining under an inverted microscope [
42,
43,
44]. The lipid accumulation was dissolved using 100% isopropanol and quantified by absorbance measurement at 520 nm using a microplate reader (Biotek model Power Wave X, Santa Clara, CA, USA). The level of lipid accumulation was expressed as inhibition percentage of adipocyte cells.
4.6.5. Assay of Anti-Inflammatory Activity
The inhibitory effect of HCA on nitric oxide (NO) production in murine macrophages like RAW 264.7 cells was evaluated using a modification of the method reported by Kaewkroek et al., 2019 [
41]. Cells were cultured in RPMI 1640 medium supplemented with sodium bicarbonate (0.1%) and glutamine 2 mM, penicillin G (100 units/mL), streptomycin (100 µg/mL), and 10% fetal calf serum. Cells were seeded at a density of 1 × 10
5 cells /well in a 96-well plate and incubated at 37 °C under 5% CO
2 for 1 h. The medium was removed and replaced with fresh medium containing 100 ng/mL of LPS and the test samples (standard, garcinia extract, optimized in situ gelling liquid formulation and blank formulation) at various concentrations. The cells were incubated for 24 h. Indomethacin was used as a positive control. The level of NO production was determined by assay of the amount of nitrile in the culture medium using Griess reagent [
45]. The optical density was measured at 570 nm using a microplate reader (Biotek model Power Wave X, Santa Clara, CA, USA). The percentage inhibition of NO production was calculated using the following equation and IC
50 values were determined (
n = 5).
where (A − C): NO
2− concentration (µM) [A: LPS (+), sample (−); B: LPS (+), sample (+); C: LPS (−), sample (−)].
4.7. Statistical Analysis
Data were presented as mean ± standard deviation (mean ± S.D.). The data were processed by Student’s t-test or one-way analysis of variance (ANOVA). p < 0.05 and p < 0.01 were regarded as statistically significant.