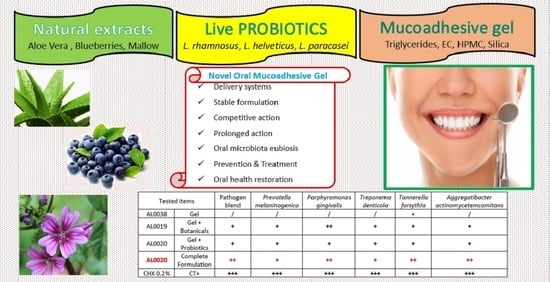
Tau-Marin Mucoadhesive Gel for Prevention and Treatment of Gum Diseases
Abstract
:1. Introduction
2. Results
2.1. Mucoadhesive Gel (AL0038) and Ingredients
2.2. Tau-Marin Mucoadhesive Gel (AL0019, AL0039, and AL0020) Preparation
2.3. Antimicrobial Activity: Antagonist Effects
2.4. Enumeration of Bacteria in Tau-Marin (AL0020) Mucoadhesive Gel by LCA and CFU
2.5. In Vitro Evaluation of Irritative Potential, Protective Efficacy, and Soothing Effect on Gingival Epithelium
2.5.1. Inserts of Gingival Epithelium Reconstructed In Vitro
2.5.2. Human IL-1 Alpha ELISA Test
2.6. In Vitro Evaluation of Irritation on Human Oral Mucosa and TEWL Measurement
- Negative control:
- Positive control:
2.7. Clinical Evaluation, Assessment of the Microbiome, and Self-Assessment Questionnaire of a Cosmetic Product for Oral Use
2.7.1. Selection of Volunteers
- (a)
- Inclusion criteria
- Caucasian subjects;
- Males and females between 18 and 70 years of age, in good general health;
- Subjects able to follow all the instructions of the study and to commit to carry out the scheduled visits for the entire duration of the study;
- Subjects who give their informed consent (Study N° RAP23816);
- Subjects with alteration of the gingival mucosa (e.g., erythema, erosions, leukoplastic lesions, and bleeding);
- Subjects with or without tartar, dental interventions, prostheses, etc.
- (b)
- Exclusion criteria
- Pregnant or nursing women;
- Subjects with a history of particular skin reactions to cosmetic products and detergents or with sensitivity to one of the components of the product;
- Subjects who are taking topical or systemic medicines that may interfere with the results of the tests (anti-inflammatory agents, cortisones, antibiotics, etc.);
- Subjects who show systemic diseases or skin disorders (eczema, psoriasis, dermatitis, etc.);
- Subjects who currently use adjuvant treatments for the well-being of gums or who have used them in the last three months before the start of this study (neither topical nor systemic);
- Diabetic subjects;
- Smokers and habitual consumers of alcoholic beverages;
- Subjects who have participated in other similar studies in the period of 30 days prior to this.
- (c)
- Drop out—reasons considered sufficient to terminate the participation of the subjects in the study:
- Free choice of subject;
- Medical reasons unrelated to treatment (e.g., onset of disease or surgery);
- Reasons related to treatment (e.g., irritation or allergic reactions).
2.7.2. Instruments and Parameters
2.7.3. Method
2.7.4. Results
2.7.5. Microbiome Analysis
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Mucoadhesive Gel (AL0038) Preparation
5.2. Tau-Marin Mucoadhesive Gel (AL0020) Preparation
5.3. Pathogenic and Probiotic Strains
5.4. Enumeration of Bacteria in Tau-Marin Mucoadhesive Gel by LCA and CFU
5.5. Number of Bacteria Released in Physiological Solution or Simulated Saliva
5.6. In Vitro Evaluation of Irritation on Human Oral Mucosa and TEWL Measurement
5.7. In Vitro Evaluation of Irritative Potential, Protective Efficacy, and Soothing Effect on Gingival Epithelium
5.8. Clinical Evaluation, Assessment of the Microbiome, and Self-Assessment Questionnaire of a Cosmetic Product for Oral Use
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AL | (Alfasigma) |
| CFUs | (Colony Forming Units) |
| HE | (Haematoxylin-Eosin-staining) |
| HOE | (Human Oral Epithelium) |
| HPMC | (Hydroxy-Propyl-Methylcellulose) |
| INCI | (International Nomenclature of Cosmetic Ingredients) |
| LCA | (Lacto-Counter Assay) |
| MCT | (Medium-Chain Triglycerides) |
| MDR | (Maximum Recovery Diluent) |
| MPs | (Modified Silicas) |
| MRS | (de Man, Rogosa and Sharpe) agar |
| MTT | (Colorimetric assay based on the enzymatic reduction of tetrazolium dye, which is chemically 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, for assessing cell metabolic activity) |
| PACs | (ProAnthoCyanidins) |
| PBS | (Phosphate Buffered Saline) |
| PS | (Physiological Solution) |
| SDS | (Sodium Dodecyl Sulphate) |
| SS | (Simulated Saliva) |
| TEWL | (TransEpidermal Water Loss) |
References
- Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030. 18 November 2022. Available online: https://www.who.int/publications/i/item/9789240061484 (accessed on 30 December 2022).
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryder, M.I.; Couch, E.T.; Chaffee, B.W. Personalized periodontal treatment for the tobacco-and alcohol-using patient. Periodontol. 2000 2018, 78, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Kurtzman, G.M.D.; Horowitz, R.A.D.; Johnson, R.; Prestiano, R.A.; Klein, B.I. The systemic oral health connection: Biofilms. Medicine 2022, 101, e30517. [Google Scholar] [CrossRef]
- Mahendra, J.; Mahendra, L.; Sharma, V.; Alamoudi, A.; Bahammam, H.A.; Mugri, M.H.; Bahammam, S.A.; Bahammam, M.A.; Zidane, B.; Nayaki, R.P.A.; et al. Red-Complex Bacterial Levels in Pregnant Women with Preeclampsia and Chronic Periodontitis. Int. Dent. J. 2022, 73, 503–510. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, M.; Liu, Y.; Luo, B.; Cui, J.; Huang, L.; Chen, K.; Liu, Y. The oral microbiota and cardiometabolic health: A comprehensive review and emerging insights. Front. Immunol. 2022, 13, e1010368. [Google Scholar]
- Jin, L. Group E. Initiator paper. Interprofessional education and multidisciplinary teamwork for prevention and effective management of periodontal disease. J. Int. Acad. Periodontol. 2015, 17 (Suppl. S1), 74–79. [Google Scholar]
- Vega-Chin, A.; de la Fuente, S.S.; Gómez-Fernández, A.; Ortiz-Acuña, L.; Mora-González, A.; Rodríguez-Masís, R.; Ramírez, K. Gingival State and Presence of Red Complex Bacteria in 12-Year-Old Schoolchildren. Int. J. Dent. Sci. 2022, 24, 161–175. [Google Scholar] [CrossRef]
- Mohanty, R.; Asopa, S.J.; Joseph, M.D.; Singh, B.; Rajguru, J.P.; Saidath, K.; Sharma, U. Red complex: Polymicrobial conglomerate in oral flora: A review. J. Family Med. Prim. Care 2019, 8, 3480–3486. [Google Scholar] [CrossRef]
- Tegegne, B.A.; Kebede, B. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 2022, 8, e09725. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Lagha, A.; LeBel, G.; Grenier, D. Dual action of highbush blueberry proanthocyanidins on Aggregatibacter actinomycetemcomitans and the host inflammatory response. BMC Complement. Altern. Med. 2018, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Yeturu, S.K.; Acharya, S.; Urala, A.S.; Pentapati, K.C. Effect of Aloe vera, chlorine dioxide, and chlorhexidine mouth rinses on plaque and gingivitis: A randomized controlled trial. J. Oral Biol. Craniofac. Res. 2016, 6, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, A.A.; Radafshar, G.; Jahandideh, Y.; Kakaei, N. Clinical evaluation of effects of local application of Aloe vera gel as an adjunct to scaling and root planning in patients with chronic periodontitis. J. Dent. 2017, 18, 165–172. [Google Scholar]
- Ipshita, S.; Kurian, I.G.; Dileep, P.; Kumar, S.; Singh, P.; Pradeep, A.R. One percent alendronate and Aloe vera gel local host modulating agents in chronic periodontitis patients with class II furcation defects: A randomized, controlled clinical trial. J. Investig. Clin. Dent. 2018, 9, e12334. [Google Scholar] [CrossRef]
- Kurian, I.G.; Dileep, P.; Ipshita, S.; Pradeep, A.R. Comparative evaluation of subgingivally-delivered 1% metformin and Aloe vera gel in the treatment of intrabony defects in chronic periodontitis patients: A randomized, controlled clinical trial. J. Investig. Clin. Dent. 2018, 9, e12324. [Google Scholar]
- AButera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]
- Giannini, G.; Ragusa, I.; Nardone, G.N.; Soldi, S.; Elli, M.; Valenti, P.; Rosa, L.; Marra, E.; Stoppoloni, D.; Merlo Pich, E.G. Probiotics-Containing Mucoadhesive Gel for Targeting the Dysbiosis Associated with Periodontal Diseases. Int. J. Dent. 2022, 2022, 50079302022. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [Green Version]
- Philip, N.; Bandara, H.M.H.N.; Leishman, S.J.; Walsh, L.J. Inhibitory effects of fruit berry extracts on Streptococcus mutans biofilms. Eur. J. Oral Sci. 2019, 127, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamath, N.P.; Tandon, S.; Nayak, R.; Naidu, S.; Anand, P.S.; Kamath, Y.S. The effect of aloe vera and tea tree oil mouthwashes on the oral health of school children. Eur. Arch. Paediatr. Dent. 2020, 21, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Karim, B.; Bhaskar, D.J.; Agali, C.; Gupta, D.; Gupta, R.K.; Jain, A.; Kanwar, A. Effect of Aloe vera mouthwash on periodontal health: Triple blind randomized control trial. Oral Health Dent. Manag. 2014, 13, 14–19. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Behbudi, G.; Mazraedoost, S.; Omidifar, N.; Gholami, A.; Chiang, W.-H.; Babapoor, A.; Pynadathu Rumjit, N. A Review on Health Benefits of Malva sylvestris L. Nutritional Compounds for Metabolites, Antioxidants, and Anti-Inflammatory, Anticancer, and Antimicrobial Applications. Evid. Based Complement. Altern. Med. 2021, 2021, 5548404. [Google Scholar]
- Gasparetto, J.C.; Martins, C.A.F.; Hayashi, S.S.; Otuky, M.F.; Pontarolo, R. Ethnobotanical and scientific aspects of Malva sylvestris L.: A millennial herbal medicine. J. Pharm. Pharmacol. 2012, 64, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]



| Commercial Name | Source | INCI | % |
|---|---|---|---|
| LABRAFAC® caprylic (C8)/Capric (C10) MCT oil | Gattefossè | Caprylic/Capric Triglyceride | 75.25 |
| Ethylcellulose | Asha Cellulose | Ethylcellulose | 5.00 |
| COMPRITOL® 888 CG | Gattefossè | Glyceryl Behenate | 1.00 |
| AEROSIL® R 972 | Evonik | Hydrophobic fumed silica/Silica dimethyl silylate | 5.00 |
| BENECEL™ K4M | Ashland | Hydroxypropyl Methylcellulose | 8.50 |
| BENECEL™ K100M | Ashland | Hydroxypropyl Methylcellulose | 5.00 |
| Mint Flavour | Farotti | - | 0.25 |
| Bacterial Strain | % | Bacteria into the Tau-Marin Gel (Theorical Title) | AL0020 (Time = 0) | |
|---|---|---|---|---|
| in 100 g gel | in 1 g gel | |||
| L. rhamnosus SP1 | 1 | 3.0 × 1011 UFC/g | 3.0 × 109 UFC/g | >4 × 109 UFC/g |
| L. helveticus SP27 | 1 | 2.0 × 1011 UFC/g | 2.0 × 109 UFC/g | |
| L. paracasei CBA-L87 | 1 | 1.0 × 1011 UFC/g | 1.0 × 109 UFC/g | |
| Commercial Name | Source | INCI | % |
|---|---|---|---|
| LABRAFAC® caprylic (C8)/Capric (C10) MCT oil | Gattefossè | Caprylic/Capric Triglyceride | 71.05 |
| Ethylcellulose | Asha Cellulose | Ethylcellulose | 5.00 |
| COMPRITOL® 888 CG | Gattefossè | Glyceryl Behenate | 1.00 |
| AEROSIL® R 972 | Evonik | Hydrophobic fumed silica/Silica dimethyl silylate | 5.00 |
| BENECEL™ K4M | Ashland | Hydroxypropyl Methylcellulose | 8.50 |
| BENECEL™ K100M | Ashland | Hydroxypropyl Methylcellulose | 5.00 |
| Aloe vera gel Podwer regular 200X | Terry Laboratories LLC | Aloe Barbadensis Leaf Extract | 0.20 |
| Blueberry (1:4) dry extract | LaBioTRE | Vaccinium Myrtillus Fruit Extract | 0.40 |
| Mallow leaves (1:4) dry extract | LaBioTRE | Malva Sylvestris Leaf Extract | 0.60 |
| Mint Flavour | Farotti | Flavour | 0.25 |
| L. rhamnosus SP1 | CSL | Lactobacillus Ferment | 1.00 |
| L. helveticus SP27 | CSL | Lactobacillus Ferment | 1.00 |
| L. paracasei CBA-L87 | CSL | Lactobacillus Ferment | 1.00 |
| Items | Pathogen Strain (s) | Against Probiotic Strain (s) |
|---|---|---|
| 1 | Five single pathogens | L. rhamnosus SP1 |
| 2 | Five single pathogens | L. paracasei CBA-L87 |
| 3 | Five single pathogens | L. helveticus SP27 |
| 4 | Five single pathogens | AL0038 (Neutral gel) |
| 5 | Five single pathogens | AL0039 (gel containing three probiotic strains) |
| 6 | Five single pathogens | AL0019 (gel containing three botanical extracts) |
| 7 | Five single pathogens | AL0020 (gel containing 3 probiotic strains + 3 extracts) |
| 8 | Five single pathogens | Aloe vera extract |
| 9 | Five single pathogens | Blueberry extract |
| 10 | Five single pathogens | Mallow extract |
| 11 | Five pathogenic bacteria (consortium) | L. rhamnosus SP1 |
| 12 | Five pathogenic bacteria (consortium) | L. paracasei CBA-L87 |
| 13 | Five pathogenic bacteria (consortium) | L. helveticus SP27 |
| 14 | Five pathogenic bacteria (consortium) | AL0038 (Neutral gel) |
| 15 | Five pathogenic bacteria (consortium) | AL0039 (gel containing three probiotic strains) |
| 16 | Five pathogenic bacteria (consortium) | AL0019 (gel containing three botanical extracts) |
| 17 | Five pathogenic bacteria (consortium) | AL0020 (gel containing 3 probiotic strains + 3 extracts) |
| 18 | Five pathogenic bacteria (consortium) | Aloe vera extract |
| 19 | Five pathogenic bacteria (consortium) | Blueberry extract |
| 20 | Five pathogenic bacteria (consortium) | Mallow extract |
| Tested Conditions in Duplicate | Pathogen Blend | P. melan | P. gingivalis | T. denticola | T. forsithia | A. actin | |
|---|---|---|---|---|---|---|---|
| DSM7089 | DSM20709 | DSM14222 | DSM102835 | DSM8324 | |||
| Uncutted well | CT− | / | / | / | / | / | / |
| Chlorexidine 0.2% | CT+ | +++ | +++ | +++ | +++ | +++ | +++ |
| AL0038 gel | Mint flavor | / | / | / | / | + | / |
| AL0039 gel | Mint flavor + three probiotics | + | + | + | + | + | + |
| AL0019 gel | Mint flavor + three botanicals | + | + | ++ | + | + | + |
| AL0020 gel | complete formulation | ++ | + | ++ | + | ++ | ++ |
| L. rhamnosus SP1 | DSM21690 | / | / | + | / | / | / |
| L. helveticus SP27 | DSM29575 | / | / | / | / | / | + |
| L. paracasei CBA-L87 | LMG-26420) | / | + | + | / | + | / |
| Aloe vera | Gel powder regular 200X | + | + | + | / | + | + |
| Blueberries (1:4) | Dry extract | + | + | / | / | / | / |
| Mallow leaves (1:4) | Dry extract | + | + | + | / | + | + |
| Time (Months) | 0 Min | 30 Min | 2 h | 5 h | 8 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 g (LCA) | 1 g (LCA) | 1 mL PS (LCA) | 1 mL PS (CFU) | 1 g (LCA) | 1 mL PS (LCA) | 1 mL PS (CFU) | 1 g (LCA) | 1 mL PS (LCA) | 1 mL PS (CFU) | 1 g (LCA) | 1 mL PS (LCA) | 1 mL PS (CFU) | |
| T0 | 5.1 × 109 | 3.9 × 109 | 8.6 × 106 | 6.7 × 106 | 2.5 × 109 | 2.0 × 108 | 8.0 × 107 | 1.3 × 109 | 8.7 × 108 | 1.3 × 108 | 1.6 × 108 | 2.3 × 109 | 5.2 × 108 |
| T1 | 5.0 × 109 | 4.3 × 109 | 8.3 × 106 | 6.3 × 106 | 2.6 × 109 | 1.6 × 108 | 7.5 × 107 | 1.5 × 109 | 8.9 × 108 | 1.8 × 108 | 1.3 × 108 | 2.8 × 109 | 6.0 × 108 |
| T3 | 5.0 × 109 | 4.5 × 109 | 9.1 × 106 | 8.4 × 106 | 2.2 × 109 | 2.5 × 108 | 9.7 × 107 | 1.2 × 109 | 1.2 × 109 | 2.3 × 108 | 1.5 × 108 | 3.0 × 109 | 6.4 × 108 |
| T6 | 4.9 × 109 | 4.0 × 109 | 3.1 × 107 | 9.5 × 106 | 1.8 × 109 | 4.5 × 108 | 1.7 × 108 | 9.2 × 108 | 2.0 × 109 | 4.8 × 108 | 1.0 × 108 | 3.3 × 109 | 8.9 × 108 |
| T9 | 4.8 × 109 | 4.0 × 109 | 4.4 × 107 | 9.1 × 106 | 1.2 × 109 | 6.3 × 108 | 1.2 × 108 | 9.0 × 108 | 2.2 × 109 | 4.9 × 108 | 1.1 × 108 | 3.6 × 109 | 8.4 × 108 |
| T12 | 4.8 × 109 | 3.9 × 109 | 4.7 × 107 | 1.0 × 107 | 1.3 × 109 | 7.3 × 108 | 1.6 × 108 | 8.6 × 108 | 2.4 × 109 | 5.6 × 108 | 1.0 × 108 | 3.9 × 109 | 8.4 × 108 |
| Time (Months) | 0 Min | 30 Min | 2 h | 5 h | 8 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 g (LCA) | 1 g (LCA) | 1 mL SS (LCA) | 1 mL SS (CFU) | 1 g (LCA) | 1 mL SS (LCA) | 1 mL SS (CFU) | 1 g (LCA) | 1 mL SS (LCA) | 1 mL SS (CFU) | 1 g (LCA) | 1 mL SS (LCA) | 1 mL SS (CFU) | |
| T0 | 5.1 × 109 | 4.1 × 109 | 9.7 × 106 | 3.9 × 106 | 2.8 × 109 | 1.3 × 108 | 6.3 × 107 | 1.6 × 109 | 7.3 × 108 | 2.5 × 108 | 1.8 × 108 | 2.6 × 109 | 6.1 × 108 |
| T1 | 5.0 × 109 | 4.1 × 109 | 9.4 × 106 | 4.4 × 106 | 2.2 × 109 | 1.8 × 108 | 5.9 × 107 | 1.3 × 109 | 8.0 × 108 | 2.0 × 108 | 1.6 × 108 | 2.8 × 109 | 6.5 × 108 |
| T3 | 5.0 × 109 | 4.3 × 109 | 1.1 × 107 | 5.9 × 106 | 2.5 × 109 | 2.8 × 108 | 7.4 × 107 | 1.3 × 109 | 9.7 × 108 | 3.1 × 108 | 1.3 × 108 | 3.0 × 109 | 7.5 × 108 |
| T6 | 4.9 × 109 | 4.0 × 109 | 3.4 × 107 | 8.9 × 106 | 2.2 × 109 | 3.8 × 108 | 9.4 × 107 | 9.3 × 108 | 1.7 × 109 | 3.8 × 108 | 1.0 × 108 | 3.5 × 109 | 8.6 × 108 |
| T9 | 4.8 × 109 | 4.1 × 109 | 3.1 × 107 | 7.9 × 106 | 1.9 × 109 | 4.3 × 108 | 1.0 × 108 | 9.2 × 108 | 2.3 × 109 | 4.0 × 108 | 9.8 × 107 | 3.7 × 109 | 7.9 × 108 |
| T12 | 4.8 × 109 | 3.8 × 109 | 4.2 × 107 | 1.1 × 107 | 1.5 × 109 | 7.3 × 108 | 1.3 × 108 | 8.5 × 108 | 2.7 × 109 | 4.7 × 108 | 9.0 × 107 | 4.0 × 109 | 8.3 × 108 |
| MTT Test—Cellular Viability (±S.D.) | ||
|---|---|---|
| Negative control (physiological solution) | 100.0% | (±6.6) |
| Positive control (SDS 0.5%) | 1.9% | (±0.1) |
| Tau-Marin gel AL0020 | 113.9% | (±8.5) |
| MTT Test—Cellular Viability (±S.D.) | ||
|---|---|---|
| Negative control (physiological solution) | 100.0% | (±6.6) |
| Positive control (SDS 0.5%) for 1 h | 1.9% | (±0.1) |
| Tau-Marin gel AL0020 + irritative agents (SDS 0.5%) for 1 h | 108.0% | (±10.9) |
| Treatment | % Cellular Viability (±S.D.) | TEWL (g/m2h) | ||
|---|---|---|---|---|
| Before Treatment | After Treatment | After Rest | ||
| Negative control | 100.0 (±2.7) | 12.9 (±0.8) | 16.8 (±0.7) | 18.8 (±1.4) |
| Positive control | 2.9 (±1.7) | 13.4 (±0.7) | 19.3 (±0.7) | 17.6 (±1.5) |
| Tau-Marin gel | 87.6 (±1.7) | 14.9 (±0.5) | 12.6 (±1.1) | 14.4 (±2.7) |
| Question | Opinion Expressed | ||||
|---|---|---|---|---|---|
| Excellent (4) | Good (3) | Sufficient (2) | Insufficient (1) | ||
| A | How do you evaluate product’s applicability? | 32% | 42% | 16% | 11% |
| B | How do you judge product’s adhesiveness? | 37% | 37% | 21% | 5% |
| C | How do you judge product’s texture? | 32% | 26% | 32% | 1% |
| D | How do you judge the taste of the product? | 26% | 21% | 53% | - |
| E | How do you judge the persistence of the flavour? | 26% | 26% | 37% | 11% |
| F | How do you judge the pleasantness of night use? | 16% | 26% | 47% | 11% |
| G | How do you rate the ease of removing the product in the morning? | 68% | 21% | 11% | - |
| H | How do you evaluate the product’s ability to relieve gum inflammation? | 53% | 47% | - | - |
| I | How do you evaluate the product’s ability to relieve gum irritation? | 58% | 42% | - | - |
| L | How do you evaluate the product’s ability to calm gingivitis? | 47% | 53% | - | - |
| M | How do you evaluate the product’s ability to calm gum bleeding? | 53% | 42% | 5% | - |
| N | How do you evaluate the product’s ability to mitigate the annoyance of heat/cold? | 37% | 58% | 5% | - |
| O | Did you feel a tingling, reddening sensation or any other alteration in the sensitivity of gum mucosae while using the product? | 100% | - | - | - |
| P | Would you buy the product again? | 26% | 37% | 26% | 11% |
| Q | How do you rate the product overall? | 21% | 63% | 16% | - |
| T0 | TF = 30 Days | Variation T0-TF | T0 vs. TF (p-Value) |
|---|---|---|---|
| Mean 1.6 (±SD 0.5) | Mean 0.7 (±SD 0.5) | −0.8 | p < 0.0001 |
| T0 | TF = 30 Days | Variation T0-TF | T0 vs. TF (p-Value) |
|---|---|---|---|
| Mean 1.5 (±SD 0.9) | Mean 0.3 (±SD 0.4) | −1.2 | p < 0.0003 |
| T0 | TF = 30 Days | T0 vs. TF (p-Value) |
|---|---|---|
| Mean 0.1 (±SD 0.3) | Mean 0.0 (±SD 0.0) | p < 0.35 |
| T0 | TF = 30 Days | T0 vs. TF (p-Value) |
|---|---|---|
| Mean 0.2 (±SD 0.5) | Mean 0.0 (±SD 0.0) | p < 0.37 |
| Items | Composition | Batch |
|---|---|---|
| Uncutted well | Negative Control (CT−) | - |
| Chlorexidine 0.2% | Positive Control (CT+) | - |
| AL0038 | Gel-mint flavour (Neutral Gel: mucoadhesive medium only) | IR0321 |
| AL0039 | Gel-mint flavour + 3 probiotics | IR0322 |
| AL0019 | Gel-mint flavour + 3 botanical extracts | IR0323 |
| AL0020 | Gel-mint flavour + 3 botanical extracts + 3 probiotics | L1538 K |
| L. rhamnosus SP1 | DSM 21690 | 22000690 |
| L. helveticus SP27 | DSM 29575 | 22003367 |
| L. paracasei CBA-L87 | LMG-26420 | 22004203 |
| Aloe vera | gel Powder regular 200X | 19007530 |
| Blueberries | (1:4) dry extract | 22003192 |
| Mallow leaves | (1:4) dry extract | 22003567 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannini, G.; Ragusa, I.; Nardone, G.N.; Soldi, S.; Elli, M.; Valenti, P.; Rosa, L. Tau-Marin Mucoadhesive Gel for Prevention and Treatment of Gum Diseases. Gels 2023, 9, 607. https://doi.org/10.3390/gels9080607
Giannini G, Ragusa I, Nardone GN, Soldi S, Elli M, Valenti P, Rosa L. Tau-Marin Mucoadhesive Gel for Prevention and Treatment of Gum Diseases. Gels. 2023; 9(8):607. https://doi.org/10.3390/gels9080607
Chicago/Turabian StyleGiannini, Giuseppe, Irene Ragusa, Giulia Nerina Nardone, Sara Soldi, Marina Elli, Piera Valenti, and Luigi Rosa. 2023. "Tau-Marin Mucoadhesive Gel for Prevention and Treatment of Gum Diseases" Gels 9, no. 8: 607. https://doi.org/10.3390/gels9080607