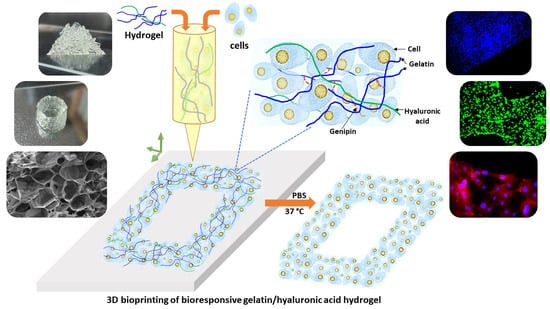
Study on Bioresponsive Gelatin-Hyaluronic Acid-Genipin Hydrogel for High Cell-Density 3D Bioprinting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gelatin-HA Hydrogel Formation and It’s Bioresponsiveness
2.2. Rheological and Mechanical Properties of the Gelatin-HA Hydrogels
2.3. Morphology of the Gelatin-HA Hydrogels
2.4. 3D Printability of the Gelatin-HA Hydrogels for Complex Structures
2.5. Cell Culture Studies on 3D Bioprinted Gelatin-HA Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrogel
4.3. Characterization of Gelatin-HA Hydrogel
4.4. 3D (bio) Printing and Four-Axis Printing
4.5. In Vitro Cytocompatibility Test
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. A 2013, 101, 272–284. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Bibi, N.; Jayawarna, V.; Thornton, P.D.; Todd, S.J.; Mart, R.J.; Smith, A.M.; Gough, J.E. Bioresponsive hydrogels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Sgambato, A.; Cipolla, L.; Russo, L. Bioresponsive Hydrogels: Chemical Strategies and Perspectives in Tissue Engineering. Gels 2016, 2, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri, S.; Ghazali, H.S.; Ghazali, Z.S.; Bhattacharyya, A.; Noh, I. Progress in biomechanical stimuli on the cell-encapsulated hydrogels for cartilage tissue regeneration. Biomater. Res. 2023, 27, 22. [Google Scholar] [CrossRef] [PubMed]
- Pourchet, L.J. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv. Healthc. Mater. 2017, 6, 1601101. [Google Scholar] [CrossRef] [PubMed]
- Lupu, A.; Gradinaru, L.M.; Gradinaru, V.R.; Bercea, M. Diversity of Bioinspired Hydrogels: From Structure to Applications. Gels 2023, 9, 376. [Google Scholar] [CrossRef]
- Niazi, M. Advanced Bioresponsive Multitasking Hydrogels in the New Era of Biomedicine. Adv. Funct. Mater. 2021, 31, 2104123. [Google Scholar] [CrossRef]
- Vu, B.; Souza, G.R.; Dengjel, J. Scaffold-free 3D cell culture of primary skin fibroblasts induces profound changes of the matrisome. Matrix Biol. Plus 2021, 11, 100066. [Google Scholar] [CrossRef]
- Daly, A.C.; Davidson, M.D.; Burdick, J.A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 2021, 12, 753. [Google Scholar] [CrossRef]
- You, S.; Xiang, Y.; Hwang, H.H.; Berry, D.B.; Kiratitanaporn, W.; Guan, J.; Yao, E.; Tang, M.; Zhong, Z.; Ma, X.; et al. High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 2023, 9, 7923. [Google Scholar] [CrossRef]
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G. Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 2017, 2, 3567–3584. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Watson, C.A.; Ranson, M. Gelapin, a degradable genipin cross-linked gelatin hydrogel. RSC Adv. 2013, 3, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, A.; Priya, V.K.; Kim, J.H.; Khatun, M.R.; Nagarajan, R.; Noh, I. Nanodiamond enhanced mechanical and biological properties of extrudable gelatin hydrogel cross-linked with tannic acid and ferrous sulphate. Biomater. Res. 2022, 26, 37. [Google Scholar] [CrossRef] [PubMed]
- Ouasti, S. Network connectivity, mechanical properties and cell adhesion for hyaluronic acid/PEG hydrogels. Biomaterials 2011, 32, 6456–6470. [Google Scholar] [CrossRef]
- Roig, F. Hyaluronan based materials with catanionic sugar-derived surfactants as drug delivery systems. Colloids Surf. B Biointerfaces 2018, 164, 218–223. [Google Scholar] [CrossRef]
- Larraneta, E. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mero, A.; Campisi, M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef] [Green Version]
- Khatun, M.R.; Bhattacharyya, A.; Taheri, S.; Ham, H.W.; Kim, H.; Chang, S.H.; Noh, I. High Molecular Weight Fucoidan Loading into and Release from Hyaluronate-Based Prefabricated Hydrogel and its Nanogel Particles Controlled by Variable Pitch and Differential Extensional Shear Technology of Advanced Twin Screw-Based System. Adv. Mater. Technol. 2023, 8, 2201478. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.; Cakić, S. A Review of Patents and Innovative Biopolymer-Based Hydrogels. Gels 2023, 9, 556. [Google Scholar] [CrossRef]
- Vasi, A.M. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Das, M.P.; Suguna, P.R.; Prasad, K.A.; Vijaylakshmi, J.V.; Renuka, M. Extraction and characterization of gelatin: A functional biopolymer. Int. J. Pharm. Pharm. Sci. 2017, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Kahoush, M. Genipin-mediated immobilization of glucose oxidase enzyme on carbon felt for use as heterogeneous catalyst in sustainable wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 105633. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Janarthanan, G.; Tran, H.N.; Ham, H.J.; Yoon, J.; Noh, I. Bioink homogeneity control during 3D bioprinting of multicomponent micro/nanocomposite hydrogel for even tissue regeneration using novel twin screw extrusion system. Chem. Eng. J. 2021, 415, 128971. [Google Scholar] [CrossRef]
- Bigia, A.; Cojazzib, G.; Panzavoltaa, S.; Roveria, N.; Rubinia, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Sung, H.W. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Ham, H.W.; Sonh, J.; Gunbayar, M.; Jeffy, R.; Nagarajan, R.; Khatun, M.R.; Noh, I. 3D bioprinting of complex tissue scaffolds with in situ homogeneously mixed alginate-chitosan-kaolin bioink using advanced portable biopen. Carbohydr. Polym. 2023, 317, 121046. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Janarthanan, G.; Kim, T.; Taheri, S.; Shin, J.; Kim, J.; Bae, H.C.; Han, H.S.; Noh, I. Modulation of bioactive calcium phosphate micro/nanoparticle size and shape during in situ synthesis of photo-crosslinkable gelatin methacryloyl based nanocomposite hydrogels for 3D bioprinting and tissue engineering. Biomater. Res. 2022, 26, 54. [Google Scholar] [CrossRef]
- Tran, H.N.; Kim, I.G.; Kim, J.H.; Chung, E.J.; Noh, I. Control of maleic acid-propylene diepoxide hydrogel for 3D printing application for flexible tissue engineering scaffold with high resolution by end capping and graft polymerization. Biomater. Res. 2022, 26, 75. [Google Scholar] [CrossRef]
- Janarthanan, G.; Lee, S.; Noh, I. 3D Printing of Bioinspired Alginate-Albumin Based Instant Gel Ink with Electroconductivity and Its Expansion to Direct Four-Axis Printing of Hollow Porous Tubular Constructs without Supporting Materials. Adv. Funct. Mater. 2021, 31, 2104441. [Google Scholar] [CrossRef]








| SI No. | Sample Composition | Sample Code |
|---|---|---|
| 1 | 0.3 g HA + 1.0 g Gelatin + 5 mg Genipin + 20 mL DW | Gel 1 |
| 2 | 0.3 g HA + 1.2 g Gelatin + 5 mg Genipin + 20 mL DW | Gel 2 |
| 3 | 0.3 g HA + 1.5 g Gelatin + 5 mg Genipin + 20 mL DW | Gel 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatun, M.R.; Bhattacharyya, A.; Gunbayar, M.; Jung, M.; Noh, I. Study on Bioresponsive Gelatin-Hyaluronic Acid-Genipin Hydrogel for High Cell-Density 3D Bioprinting. Gels 2023, 9, 601. https://doi.org/10.3390/gels9080601
Khatun MR, Bhattacharyya A, Gunbayar M, Jung M, Noh I. Study on Bioresponsive Gelatin-Hyaluronic Acid-Genipin Hydrogel for High Cell-Density 3D Bioprinting. Gels. 2023; 9(8):601. https://doi.org/10.3390/gels9080601
Chicago/Turabian StyleKhatun, Mst Rita, Amitava Bhattacharyya, Maral Gunbayar, Minsik Jung, and Insup Noh. 2023. "Study on Bioresponsive Gelatin-Hyaluronic Acid-Genipin Hydrogel for High Cell-Density 3D Bioprinting" Gels 9, no. 8: 601. https://doi.org/10.3390/gels9080601