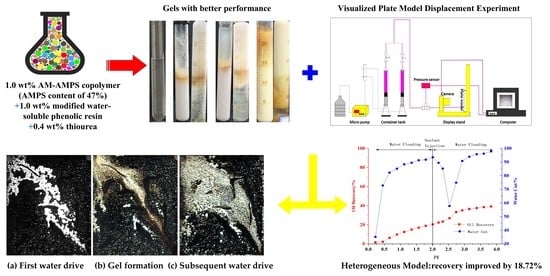
Study on Water-Soluble Phenolic Resin Gels for High-Temperature and High-Salinity Oil Reservoir
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Gelation Performances of the Modified Soluble Phenolic Resin Gels
2.1.1. Gelation Time of the Modified Soluble Phenolic Resin Gels
2.1.2. Storage Moduli of the Modified Soluble Phenolic Resin Gels
2.1.3. Long-Term Stability of the Modified Soluble Phenolic Resin Gels
2.2. Evaluation of Plugging Performance of Modified Water-Soluble Phenolic Resin Gel
2.2.1. Gel Strength after Adding Water
2.2.2. Plugging Ability of Gel in Porous Media
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of the AM-AMPS Copolymer Solution
4.2.2. Synthesis Method of the Modified Water-Soluble Phenolic Resin
4.2.3. Preparation Method of Water-Soluble Phenolic Resin Gel
4.2.4. Determination of Gelation Time and Strength of Gel
4.2.5. Method for Determining the Syneresis Rate of Gel
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xin, X.; Liu, Q.; Liu, S.; Yu, G.; Wan, Q. Optimization of Gel Flooding during the High Water Cut Stage in a Conglomerate Reservoir of the Xinjiang A Oilfield. Polymers 2023, 15, 1809. [Google Scholar] [CrossRef]
- Ge, J.; Zheng, W.; Wei, K.; Deng, X.; Fang, Q. Preparation and Performance Evaluation of Phenolic Gel Using for Plugging Cracks in Honghe Reservoir. Oilfield Chem. 2020, 37, 432–437. [Google Scholar] [CrossRef]
- Wu, Q.; Ge, J.; Zhang, G.; Guo, H.; Zhao, Q. The water shutoff simulation experiment using tube for high-strength plugging agent in fractured reservior. Acta Pet. Sin. 2019, 40, 1368–1375. [Google Scholar]
- Zhou, R.; Zhang, D.; Wei, J. Experiment on the profile control effect of different strength gel systems in heterogeneous reservoir. Energy Rep. 2021, 7, 6023–6030. [Google Scholar] [CrossRef]
- Ding, F.; Dai, C.; Sun, Y.; Zhao, G.; You, Q.; Liu, Y. Gelling Behavior of PAM/Phenolic Crosslinked Gel and Its Profile Control in a Low-Temperature and High-Salinity Reservoir. Gels 2022, 8, 433. [Google Scholar] [CrossRef]
- Lv, Q.; Ge, J.; Guo, H.; Chen, J.; Fan, J.; Mao, Y. Development of Phenolic Gel for Profile Control under High Temperature and High Salt Condition. J. Xi’an Shiyou Univ. (Nat. Sci. Ed.) 2023, 38, 61–67+112. [Google Scholar]
- Yang, Q.; Zhao, M.; Gao, M.; Song, X.; Dai, C. The experimental study of silica nanoparticles strengthened polymer gel system. J. Dispers. Sci. Technol. 2019, 42, 298–305. [Google Scholar] [CrossRef]
- Unomah, M.; Thach, S.; Shong, R.; App, J.; Zhang, T.; Kim, D.H.; Malik, T.; Dwarakanath, V. Performance of conformance gels under harsh conditions. In Proceedings of the SPE Improved Oil Recovery Conference, Oklahoma City, OK, USA, 14–18 April 2018. [Google Scholar]
- Juárez, J.L.; Rodriguez, M.R.; Montes, J.; Trujillo, F.D.; Monzòn, J.; Dupuis, G.; Gaillard, N. Conformance gel design for high temperature reservoirs. In Proceedings of the SPE Europec, Online, 1–3 December 2020. [Google Scholar]
- Guo, N.; Wu, J.; Li, L.; Zhang, R.; Liao, X.; Chen, L. Study on preparation and stability mechanism of water plugging gel with high performance for Tahe Oilfield. Appl. Chem. 2020, 49, 3011–3015. [Google Scholar] [CrossRef]
- Ge, J.; Guo, H.; Zhang, T.; Zhou, D.; Xu, Y.; Lv, Q. Development of temperature and salinity resistant phenolic gel and its performance regulation mechanism. Acta Pet. Sin. 2022, 43, 1145–1157. [Google Scholar]
- Chen, L.; Zhang, G.; Ge, J.; Jiang, P.; Zhu, X.; Lin, Y.; Han, S. A Novel Thermal-Resistance and Salt-Tolerance Gel with Low-Concentration Crosslinkers for Water Shutoff in Tahe Oilfield. In Proceedings of the SPE Asia Pacific Unconventional Resources Conference and Exhibition, Brisbane, Australia, 9–11 November 2015. [Google Scholar]
- Wang, J.; Luo, P. Development of the new associative polymer in profile control. Oil Drill. Prod. Technol. 2000, 3, 54–56+85. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Wang, K.; Zhao, M.; Zhao, G.; Shuai, Y.; Yan, Z.; You, Q. New insights into the hydroquinone (HQ)–hexamethylenetetramine (HMTA) gel system for water shut-off treatment in high temperature reservoirs. J. Ind. Eng. Chem. 2016, 35, 20–28. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Ge, J.; Guo, H.; Wu, Q.; Mao, Y. Phenolic resin gel suitable for medium-temperature and high-salinity reservoirs. J. Mol. Liq. 2022, 364, 119887. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Wang, Y.; Xu, X.; Zhu, Y.; Li, R. Gelation Performance and Microstructure Study of Chromium Gel and Phenolic Resin Gel in Bulk and Porous Media. J. Energy Resour. Technol.-Trans. Asme. 2014, 136, 042910. [Google Scholar] [CrossRef]
- Shang, X.; Pu, C.; Wu, F.; Liu, J. Laboratory study on the high strength gel system. Appl. Chem. 2011, 40, 1911–1914. [Google Scholar] [CrossRef]
- Cui, C.; Zhou, Z.; He, Z. Enhance oil recovery in low permeability reservoirs: Optimization and evaluation of ultra-high molecular weight HPAM/phenolic weak gel system. J. Pet. Sci. Eng. 2020, 195, 107908. [Google Scholar] [CrossRef]
- Zhao, G.; Dai, C.; Chen, A.; Yan, Z.; Zhao, M. Experimental study and application of gels formed by nonionic polyacrylamide and phenolic resin for in-depth profile control. J. Pet. Sci. Eng. 2015, 135, 552–560. [Google Scholar] [CrossRef]
- Gu, C.; Lv, Y.; Fan, X.; Zhao, C.; Dai, C.; Zhao, G. Study on rheology and microstructure of phenolic resin cross-linked nonionic polyacrylamide (NPAM) gel for profile control and water shutoff treatments. J. Pet. Sci. Eng. 2018, 169, 546–552. [Google Scholar] [CrossRef]
- Yu, H.; Ma, Z.; Tang, L.; Li, Y.; Shao, X.; Tian, Y.; Qian, J.; Fu, J.; Li, D.; Wang, L.; et al. The Effect of Shear Rate on Dynamic Gelation of Phenol Formaldehyde Resin Gel in Porous Media. Gels 2022, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ge, J.; Li, L.; Zhang, G.; Li, Z.; Wang, W.; Liu, M. New insights and experimental investigation of high-temperature gel reinforced by nano-SiO2. Gels 2022, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. Study on Phenolic Resin Gel for Plugging Fractures in Honghe Oilfield. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2022. [Google Scholar]
- Xu, Y.; Ge, J.; Guo, H.; Wei, K. The Gelation Law of Partially Hydrolyzed Polyacrylamide-Phenol-Formaldehyde Resin Under Medium-High Temperature and Medium-High Salinity Conditions. Acta Pet. Sin. 2022, 38, 1336. [Google Scholar]
- Qu, J.; Wang, P.; You, Q.; Zhao, G.; Sun, Y.; Liu, Y. Soft Movable Polymer Gel for Controlling Water Coning of Horizontal Well in Offshore Heavy Oil Cold Production. Gels 2022, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.; Liu, Y.; Chen, J.; Bo, L.; Qu, G.; Jiang, N.; He, W. Preparation and Performance Evaluation of a Temperature and Salt Resistant Hydrophobic Associative Weak Polymer Gel System. Molecules 2023, 28, 3125. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, J.; Dong, R.; Wang, J.; Yang, Z. Modification of Phenolic Prepolymer Crosslinking Agent and Evaluation of Glue-forming Properties. Shandong Chem. Ind. 2020, 49, 24–26. [Google Scholar] [CrossRef]
- Zhang, W. Study on High Temperature and High Salt Tolerance of Gel in Tahe Oilfield. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2018. [Google Scholar]
- Guo, H.; Ge, J.; Xu, Y.; Lv, Q.; Li, Z.; Zhou, D.; Tao, Z. Preparation and Mechanism of Stability for High-Temperature and High-Salinity Gels. SPE J. 2022, 27, 3565–3578. [Google Scholar] [CrossRef]
- Qiu, L.; Pu, W.; Zhang, J. Synthesis and Gelling Performance Evaluation of an Improved Phenolic Resin Crosslinker. Adv. Fine Petrochem. 2011, 12, 15–18. [Google Scholar]
- Sun, L.; Li, M.; Lin, M.; Guo, J.; Peng, B.; Dong, Z. Synthesis and Hydration Properties of Water Soluble Phenol-formaldehyde Resin. Chin. J. Appl. Chem. 2008, 25, 961–965. [Google Scholar]
- Chen, S.; Zhao, K.; Chang, D. Synthesis of phenolic resin prepolymer plugging agent with strength for oil well plugging. China Synth. Resin Plast. 2015, 32, 30–33. [Google Scholar]
- Sydansk, R.D. A New Conformance-Improvement-Treatment Chromium (III) Gel Technology. In Proceedings of the SPE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 17–20 April 1988. [Google Scholar]
- Ran, Y.; Zhang, G.; Jiang, P.; Pei, H. Preparation Method and Performance Evaluation of a Gel Based on AM/AMPS Copolymer. Gels 2022, 8, 802. [Google Scholar] [CrossRef] [PubMed]








| Instrument Name | Instrument Type | Manufacturer |
|---|---|---|
| Precision digital display mixer | JJ-1 | Jintan Jincheng Guosheng Experimental Instrument Factory (Changzhou, China) |
| Electronic balance | ME403 | Mettler Toledo International Trade Co., Ltd. (Shanghai, China) |
| Projector display stand | GK-8000A | Ruiying Information Technology Co., Ltd. (Guangzhou, China) |
| Micro injection pump | 100DX | Teledyne Isco, Inc. (Lincoln, Nebraska, USA) |
| Alcohol blowtorch | GW-6 | Subei Experimental Instrument Co., Ltd. (Taizhou, China) |
| Constant temperature oven | DHG-9070A | Jinghong Experimental Equipment Co., Ltd. (Shanghai, China) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, Y.; Zhang, G.; Jiang, P.; Pei, H. Study on Water-Soluble Phenolic Resin Gels for High-Temperature and High-Salinity Oil Reservoir. Gels 2023, 9, 489. https://doi.org/10.3390/gels9060489
Ran Y, Zhang G, Jiang P, Pei H. Study on Water-Soluble Phenolic Resin Gels for High-Temperature and High-Salinity Oil Reservoir. Gels. 2023; 9(6):489. https://doi.org/10.3390/gels9060489
Chicago/Turabian StyleRan, Yunling, Guicai Zhang, Ping Jiang, and Haihua Pei. 2023. "Study on Water-Soluble Phenolic Resin Gels for High-Temperature and High-Salinity Oil Reservoir" Gels 9, no. 6: 489. https://doi.org/10.3390/gels9060489