Rheological Characteristics of Soluble Cress Seed Mucilage and β-Lactoglobulin Complexes with Salts Addition: Rheological Evidence of Structural Rearrangement
Abstract
:1. Introduction
2. Results and Discussion
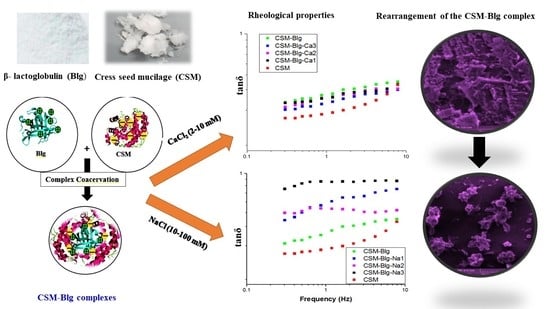
2.1. Morphological Analysis and Steady Shear
2.2. Frequency Sweep Results
2.3. Dilute Solution Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Cress Seed Mucilage Extraction and Purification
4.3. Preparation of CSM–Blg Complexes
4.4. Rheology
4.4.1. Steady Shear Values
4.4.2. Oscillatory Measurements
4.4.3. Intrinsic Viscosity
4.4.4. Hydrodynamic Coil and Volume Radius, Stiffness Parameter
4.5. Morphological Observation
4.6. Fourier-Transform Infrared (FTIR) Spectroscopy
4.7. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Reuhs, B.L.; Jones, O.G. Influence of PEGylation on the ability of carboxymethyl-dextran to form complexes with α-lactalbumin. Food Chem. 2016, 196, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Kashaninejad, M. Rheological properties for determining the interaction of soluble cress seed mucilage and β-lactoglobulin nanocomplexes under sucrose and lactose treatments. Food Chem. 2022, 378, 132133. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chai, J.; Zhang, T.; Yuan, Y.; Saini, R.K.; Xu, M.; Li, S.; Shang, X. Phase behavior, thermodynamic and rheological properties of ovalbumin/dextran sulfate: Effect of biopolymer ratio and salt concentration. Food Hydrocoll. 2021, 118, 106777. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.B.; Chen, B.; Rao, J. Phase behavior and complex coacervation of concentrated pea protein isolate-beet pectin solution. Food Chem. 2020, 307, 125536. [Google Scholar] [CrossRef]
- Hasanvand, E.; Rafe, A. Rheological and structural properties of rice bran protein-flaxseed (Linum usitatissimum L.) gum complex coacervates. Food Hydrocoll. 2018, 83, 296–307. [Google Scholar] [CrossRef]
- Taheri, A.; Kashaninejad, M.; Tamaddon, A.M.; Jafari, S.M. Comparison of binary cress seed mucilage (CSM)/β-lactoglobulin (BLG) and ternary CSG-BLG-Ca (calcium) complexes as emulsifiers: Interfacial behavior and freeze-thawing stability. Carbohydr. Polym. 2021, 266, 118148. [Google Scholar] [CrossRef]
- Schestkowa, H.; Wollborn, T.; Westphal, A.; Wagemans, A.M.; Fritsching, U.; Drusch, S. Conformational state and charge determine the interfacial stabilization process of beta-lactoglobulin at preoccupied interfaces. J. Colloid Interface Sci. 2019, 536, 300–309. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Gao, J.; Cao, M.; Ren, G.; Xie, H.; Yao, M. Formation and Characterization of β-Lactoglobulin and Gum Arabic Complexes: The Role of pH. Molecules 2020, 25, 3871. [Google Scholar] [CrossRef]
- Ramamirtham, S.; Williams, M.A.K.; Zare, D.; Weeks, M.; Whitby, C.P. Complexes of β-lactoglobulin and high methyl-esterified pectin as a one-shot delivery system for reinforcing oil/water interfaces. Soft Matter 2021, 17, 8517–8522. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, J.; Zhang, H.; Nishinari, K.; Fang, Y. Electrostatic complexation of β-lactoglobulin aggregates with κ-carrageenan and the resulting emulsifying and foaming properties. J. Dairy Sci. 2020, 103, 8709–8720. [Google Scholar] [CrossRef]
- Taheri, A.; Kashaninejad, M.; Tamaddon, A.M.; Ganjeh, M.; Jafari, S.M. Complexation of cress seed mucilage and β-lactoglobulin; optimization through response surface methodology and adaptive neuro-fuzzy inference system (ANFIS). Chemom. Intell. Lab. Syst. 2022, 228, 104615. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Khezerlou, A.; Jafari, S.M.; Pilevar, Z.; Mortazavian, A.M. Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci. 2020, 280, 102164. [Google Scholar] [CrossRef] [PubMed]
- Fahami, A.; Fathi, M. Development of cress seed mucilage/PVA nanofibers as a novel carrier for vitamin A delivery. Food Hydrocoll. 2018, 81, 31–38. [Google Scholar] [CrossRef]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Razmkhah, S.; Razavi, S.M.A.; Mohammadifar, M.A. Purification of cress seed (Lepidium sativum) gum: A comprehensive rheological study. Food Hydrocoll. 2016, 61, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shim, Y.Y.; Reaney, M.J.T. Ionic strength and hydrogen bonding effects on whey protein isolate–flaxseed gum coacervate rheology. Food Sci. Nutr. 2020, 8, 2102–2111. [Google Scholar] [CrossRef] [Green Version]
- Fitch, R.M.; Tsai, C.H. Particle Formation in Polymer Colloids, III: Prediction of the Number of Particles by a Homogeneous Nucleation Theory. In Polymer Colloids: Proceedings of an American Chemical Society Symposium on Polymer Colloids held in Chicago, IL, USA, 13–18 September 1970; Plenum Press: New York, NY, USA, 1971; pp. 73–102. [Google Scholar]
- Xin, X.; Wang, L.; Shen, J.; Xu, G.; Li, Y. Rheological properties of hydrolyzed polyacrylamide/sodium oleate mixed system in the presence of different inorganic salts. J. Pet. Sci. Eng. 2014, 114, 15–21. [Google Scholar] [CrossRef]
- Goh, K.K.T.; Teo, A.; Sarkar, A.; Singh, H. Milk protein-polysaccharide interactions. In Milk Proteins: From Expression to Food; Elsevier: Amsterdam, The Netherlands, 2019; pp. 499–535. ISBN 9780128152515. [Google Scholar]
- Tavares, L.; Noreña, C.P.Z. Encapsulation of garlic extract using complex coacervation with whey protein isolate and chitosan as wall materials followed by spray drying. Food Hydrocoll. 2019, 89, 360–369. [Google Scholar] [CrossRef]
- Fan, C.; Han, S.; Liu, F.; Liu, Y.; Wang, L.; Pan, S. Influence of calcium lactate and pH on emulsification of low-methoxylated citrus pectin in a Pickering emulsion. J. Dispers. Sci. Technol. 2017, 38, 1175–1180. [Google Scholar] [CrossRef]
- Yan, J.N.; Xue, S.; Du, Y.N.; Wang, Y.Q.; Xu, S.Q.; Wu, H.T. Influence of pH and blend ratios on the complex coacervation and synergistic enhancement in composite hydrogels from scallop (patinopecten yessoensis) protein hydrolysates and κ-carrageenan/xanthan gum. LWT 2022, 154, 112745. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Shen, J.; Wang, Y.; Reaney, M.J.T. Whey protein isolate and flaxseed (Linum usitatissimum L.) gum electrostatic coacervates: Turbidity and rheology. Food Hydrocoll. 2017, 64, 18–27. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; McClements, D.J.; Li, T. Impact of alginate block type on the structure and physicochemical properties of curcumin-loaded complex biopolymer nanoparticles. LWT 2022, 162, 113435. [Google Scholar] [CrossRef]
- Wang, X.; Lee, J.; Wang, Y.W.; Huang, Q. Composition and rheological properties of β-lactoglobulin/pectin coacervates: Effects of salt concentration and initial protein/polysaccharide ratio. Biomacromolecules 2007, 8, 992–997. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeganathan, B.; Dong, H.; Chen, L.; Vasanthan, T. Effect of sodium chloride on the thermodynamic, rheological, and microstructural properties of field pea protein isolate/chitosan complex coacervates. Food Chem. 2021, 344, 128569. [Google Scholar] [CrossRef]
- Tavares, L.; Souza, H.K.S.; Gonçalves, M.P.; Rocha, C.M.R. Physicochemical and microstructural properties of composite edible film obtained by complex coacervation between chitosan and whey protein isolate. Food Hydrocoll. 2021, 113, 106471. [Google Scholar] [CrossRef]
- Wang, W.; Shen, M.; Liu, S.; Jiang, L.; Song, Q.; Xie, J. Gel properties and interactions of Mesona blumes polysaccharide-soy protein isolates mixed gel: The effect of salt addition. Carbohydr. Polym. 2018, 192, 193–201. [Google Scholar] [CrossRef]
- Liberman, G.N.; Ochbaum, G.; Bitton, R.; Arad, S.M. Antimicrobial hydrogels composed of chitosan and sulfated polysaccharides of red microalgae. Polymer 2021, 215, 123353. [Google Scholar] [CrossRef]
- Li, G.Y.; Chen, Q.H.; Su, C.R.; Wang, H.; He, S.; Liu, J.; Nag, A.; Yuan, Y. Soy protein-polysaccharide complex coacervate under physical treatment: Effects of pH, ionic strength and polysaccharide type. Innov. Food Sci. Emerg. Technol. 2021, 68, 102612. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.B.; Chen, B.; Rao, J. Microencapsulation of hemp seed oil by pea protein isolate-sugar beet pectin complex coacervation: Influence of coacervation pH and wall/core ratio. Food Hydrocoll. 2021, 113, 106423. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, M.; Devahastin, S. Color/aroma changes of 3D-Printed buckwheat dough with yellow flesh peach as triggered by microwave heating of gelatin-gum Arabic complex coacervates. Food Hydrocoll. 2021, 112, 106358. [Google Scholar] [CrossRef]
- Li, K.Y.; Zhang, X.R.; Huang, G.Q.; Teng, J.; Guo, L.P.; Li, X.D.; Xiao, J.X. Complexation between ovalbumin and gum Arabic in high total biopolymer concentrations and the emulsifying ability of the complexes. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 642, 128624. [Google Scholar] [CrossRef]
- Weinbreck, F.; Minor, M.; De Kruif, C.G. Microencapsulation of oils using whey protein/gum arabic coacervates. J. Microencapsul. 2004, 21, 667–679. [Google Scholar] [CrossRef]
- Xiong, W.; Ren, C.; Jin, W.; Tian, J.; Wang, Y.; Shah, B.R.; Li, J.; Li, B. Ovalbumin-chitosan complex coacervation: Phase behavior, thermodynamic and rheological properties. Food Hydrocoll. 2016, 61, 895–902. [Google Scholar] [CrossRef]
- Xiong, W.; Ren, C.; Tian, M.; Yang, X.; Li, J.; Li, B. Complex coacervation of ovalbumin-carboxymethylcellulose assessed by isothermal titration calorimeter and rheology: Effect of ionic strength and charge density of polysaccharide. Food Hydrocoll. 2017, 73, 41–50. [Google Scholar] [CrossRef]
- Benoso, P.; Bittante, A.M.Q.B.; Moraes, I.C.F.; do Amaral Sobral, P.J. Rheological and viscoelastic properties of colloidal solutions based on gelatins and chitosan as affected by pH. Int. J. Food Sci. Technol. 2022, 57, 2365–2375. [Google Scholar] [CrossRef]
- Razi, S.M.; Motamedzadegan, A.; Shahidi, S.A.; Rashidinejad, A. Steady and dynamic shear rheology as a toolfor evaluation of the interactions between egg white albumin and basil seed gum. Rheol. Acta 2020, 59, 317–331. [Google Scholar] [CrossRef]
- Kan, L.; Capuano, E.; Oliviero, T.; Renzetti, S. Wheat starch-tannic acid complexes modulate physicochemical and rheological properties of wheat starch and its digestibility. Food Hydrocoll. 2022, 126, 107459. [Google Scholar] [CrossRef]
- Hammani, S.; Moulai-Mostefa, N.; Samyn, P.; Bechelany, M.; Dufresne, A.; Barhoum, A. Morphology, Rheology and Crystallization in Relation to the Viscosity Ratio of Polystyrene/Polypropylene Polymer Blends. Materials 2020, 13, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Shah, L.A.; Rehman, T.; Khan, A.; Iqbal, A.; Ullah, M.; Alam, S. Synthesis of physically cross-linked gum Arabic-based polymer hydrogels with enhanced mechanical, load bearing and shape memory behavior. Iran. Polym. J. (Engl. Ed.) 2020, 29, 351–360. [Google Scholar] [CrossRef]
- Ivancic, R.J.S.; Orski, S.V.; Audus, D.J. Structure-Dilute Solution Property Relationships of Comblike Macromolecules in a Good Solvent. Macromolecules 2022, 55, 766–775. [Google Scholar] [CrossRef]
- Jo, W.; Bak, J.H.; Yoo, B. Rheological characterizations of concentrated binary gum mixtures with xanthan gum and galactomannans. Int. J. Biol. Macromol. 2018, 114, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Lupo, C.; Boulos, S.; Nyström, L. Influence of Partial Acid Hydrolysis on Size, Dispersity, Monosaccharide Composition, and Conformation of Linearly-Branched Water-Soluble Polysaccharides. Molecules 2020, 25, 2982. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Yang, L.; Gu, J.; Cao, Y.; Li, Z.; Xu, J.; Xue, C. Kinetic interactions of nanocomplexes between astaxanthin esters with different molecular structures and β-lactoglobulin. Food Chem. 2021, 335, 127633. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Kou, M.; Fan, J.; Pan, W.; Feng, Z.J.; Su, Y.; Yang, Y.; Zhou, W. Structural characteristics and rheological properties of ovalbumin-gum arabic complex coacervates. Food Chem. 2018, 260, 1–6. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Reversed chitosan-alginate polyelectrolyte complex for stability improvement of alpha-amylase: Optimization and physicochemical characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef]
- Raei, M.; Rafe, A.; Shahidi, F. Rheological and structural characteristics of whey protein-pectin complex coacervates. J. Food Eng. 2018, 228, 25–31. [Google Scholar] [CrossRef]
- Hosseini, S.M.H.; Emam-Djomeh, Z.; Razavi, S.H.; Moosavi-Movahedi, A.A.; Saboury, A.A.; Atri, M.S.; Van der Meeren, P. β-Lactoglobulin-sodium alginate interaction as affected by polysaccharide depolymerization using high intensity ultrasound. Food Hydrocoll. 2013, 32, 235–244. [Google Scholar] [CrossRef]
- Taheri, A.; Kashaninejad, M.; Tamaddon, A.M.; Jafari, S.M. Vitamin D3 cress seed mucilage -β-lactoglobulin nanocomplexes: Synthesis, characterization, encapsulation and simulated intestinal fluid in vitro release. Carbohydr. Polym. 2020, 256, 117420. [Google Scholar] [CrossRef]
- Behrouzian, F.; Razavi, S.M.A.; Karazhiyan, H. Intrinsic viscosity of cress (Lepidium sativum) seed gum: Effect of salts and sugars. Food Hydrocoll. 2014, 35, 100–105. [Google Scholar] [CrossRef]
- Tiwari, P.; Ranjan, R.; Das, K.; Bohidar, H.; Aswal, V.K.; Arfin, N. Exfoliation and gelation in laponite-carboxymethyl cellulose complexes and its application in sustained drug release. Polym. Bull. 2019, 77, 5389–5406. [Google Scholar] [CrossRef]
- Escócio, V.A.; Pacheco, E.B.A.V.; Silva, A.L.N.D.; Cavalcante, A.D.P.; Visconte, L.L.Y. Rheological Behavior of Renewable Polyethylene (HDPE) Composites and Sponge Gourd (Luffa cylindrica) Residue. Int. J. Polym. Sci. 2015, 2015, 714352. [Google Scholar] [CrossRef] [Green Version]
- Bastos, L.P.H.; de Carvalho, C.W.P.; Garcia-Rojas, E.E. Formation and characterization of the complex coacervates obtained between lactoferrin and sodium alginate. Int. J. Biol. Macromol. 2018, 120, 332–338. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.G.; Cifre, J.G.H. Hydrodynamic Properties of Biomacromolecules and Macromolecular Complexes: Concepts and Methods. A Tutorial Mini-Review. J. Mol. Biol. 2020, 432, 2930–2948. [Google Scholar] [CrossRef] [PubMed]
- Smidsrød, O.; Haug, A. Estimation of the relative stiffness of the molecular chain in polyelectrolytes from measurements of viscosity at different ionic strengths. Biopolymers 1971, 10, 1213–1227. [Google Scholar] [CrossRef] [PubMed]







| Model | CaCl2 (mM) | NaCl (mM) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | 0 | 10 | 50 | 100 | |
| CSM–Blg | CSM–Blg–Ca1 | CSM–Blg–Ca2 | CSM–Blg–Ca3 | CSM–Blg | CSM–Blg–Na1 | CSM–Blg–Na2 | CSM–Blg–Na3 | |
| Power law | ||||||||
| kp (Pa·sn) | 3.48 ± 0.126 d | 4.5 ± 0.084 c | 4.92 ± 0.133 b | 7.15 ± 0.152 a | 3.48 ± 0.126 b | 2.37 ± 0.110 d | 7.02 ± 0.148 a | 2.70 ± 0.110 c |
| np | 0.326 ± 0.007 a | 0.214 ± 0.002 b | 0.221 ± 0.002 b | 0.163 ± 0.001 c | 0.326 ± 0.007 a | 0.253 ± 0.003 c | 0.278 ± 0.004 b | 0.248 ± 0.002 c |
| R2 | 0.982 | 0.866 | 0.894 | 0.771 | 0.982 | 0.914 | 0.897 | 0.924 |
| Bingham | ||||||||
| τ0 B (Pa) | 2.81 ± 0.086 d | 3.60 ± 0.314 c | 4.10 ± 0.152 b | 6.17 ± 0.189 a | 2.81 ± 0.086 b | 1.84 ± 0.113 d | 6.80 ± 0.157 a | 2.1 ± 0.113 c |
| ηB (Pa s) | 0.212 ± 0.002 a | 0.171 ± 0.001 c | 0.171 ± 0.002 c | 0.181 ± 0.001 b | 0.212 ± 0.002 a | 0.114 ± 0.002 c | 0.212 ± 0.003 a | 0.124 ± 0.001 b |
| R2 | 0.925 | 0.951 | 0.952 | 0.884 | 0.925 | 0.961 | 0.901 | 0.955 |
| Herschel-Bulkley | ||||||||
| kH (Pa·sn) | 1.05 ± 0.010 d | 3.22 ± 0.032 c | 4.18 ± 0.018 b | 5.30 ± 0.024 a | 1.05 ± 0.010 d | 1.15 ± 0.011 c | 2.89 ± 0.033 a | 1.71 ± 0.020 b |
| nH | 0.761 ± 0.003 a | 0.162 ± 0.002 c | 0.491 ± 0.003 b | 0.491 ± 0.002 b | 0.761 ± 0.003 a | 0.342 ± 0.001 c | 0.421 ± 0.002 b | 0.336 ± 0.001 d |
| R2 | 0.988 | 0.970 | 0.960 | 0.888 | 0.988 | 0.961 | 0.902 | 0.965 |
| Casson | ||||||||
| τ0 c (Pa) | 1.28 ± 0.015 d | 1.64 ± 0.028 c | 1.74 ± 0.041 b | 2.24 ± 0.025 a | 1.28 ± 0.015 b | 1.13 ± 0.012 c | 2.15 ± 0.010 a | 1.22 ± 0.018 bc |
| ηc(Pa s) | 0.360 ± 0.005 a | 0.273 ± 0.004 b | 0.272 ± 0.001 b | 0.243 ± 0.001 c | 0.360 ± 0.005 a | 0.239 ± 0.004 d | 0.321 ± 0.003 b | 0.246 ± 0.002 c |
| R2 | 0.970 | 0.958 | 0.927 | 0.808 | 0.970 | 0.954 | 0.881 | 0.949 |
| Treatments | k′ | n′ | R2 | k″ | n″ | R2 | K″/k′ | α |
|---|---|---|---|---|---|---|---|---|
| CaCl2 (mM) | ||||||||
| 0 | 2.66 ± 0.03 d | 0.140 ± 0.003 a | 0.956 | 0.77 ± 0.003 c | 0.292 ± 0.002 a | 0.991 | 0.290 ± 0.002 a | 0.631 ± 0.005 b |
| 2 | 3.15 ± 0.01 c | 0.125 ± 0.001 b | 0.973 | 0.82 ± 0.002 c | 0.278 ± 0.002 b | 0.992 | 0.266 ± 0.001 c | 0.659 ± 0.004 a |
| 5 | 3.51 ± 0.02 b | 0.112 ± 0.001 c | 0.950 | 0.98 ± 0.004 b | 0.212 ± 0.001 c | 0.980 | 0.280 ± 0.002 b | 0.516 ± 0.006 d |
| 10 | 4.35 ± 0.04 a | 0.096 ± 0.002 d | 0.991 | 1.23 ± 0.08 a | 0.189 ± 0.001 d | 0.983 | 0.283 ± 0.003 b | 0.597 ± 0.003 c |
| NaCl (mM) | ||||||||
| 0 | 2.66 ± 0.03 a | 0.140 ± 0.003 b | 0.956 | 0.77 ± 0.003 b | 0.292 ± 0.002 b | 0.991 | 0.290 ± 0.002 d | 0.631 ± 0.005 c |
| 10 | 1.37 ± 0.01 c | 0.134 ± 0.001 c | 0.987 | 0.70 ± 0.001 d | 0.311 ± 0.003 a | 0.987 | 0.513 ± 0.001 b | 1.25 ± 0.004 a |
| 50 | 1.82 ± 0.01 b | 0.148 ± 0.003 a | 0.985 | 0.84 ± 0.001 a | 0.148 ± 0.002 c | 0.954 | 0.464 ± 0.001 c | 0.452 ± 0.002 d |
| 100 | 0.880 ± 0.002 d | 0.105 ± 0.002 d | 0.993 | 0.73 ± 0.002 c | 0.137 ± 0.001 d | 0.948 | 0.821 ± 0.002 a | 1.06 ± 0.002 b |
| Sample | Huggins | Kramer | Tanglertpaibul & Rao | Higiro 1 | Rcoil (nm) | Vcoil (nm3) |
|---|---|---|---|---|---|---|
| CSM–Blg | R2 = 0.91 η = 0.97 ± 0.008 | R2 = 0.88 η = 0.72 ± 0.004 | R2 = 0.97 η = 0.42 ± 0.003 | R2 = 0.98 η = 0.46 ± 0.003 a | 8.50 ± 1.00 a | 2662.0 ± 74.2 a |
| CSM–Blg–Ca1 | R2 = 0.89 η = 2.11 ± 0.21 | R2 = 0.91 η = 1.80 ± 0.21 | R2 = 0.95 η = 0.58 ± 0.003 | R2 = 0.96 η = 0.32 ± 002 b | 7.76 ± 0.51 ab | 1956.2 ± 58.7 b |
| CSM–Blg–Ca2 | R2 = 0.91 η = 1.80 ± 0.15 | R2 = 0.93 η = 1.62 ± 0.14 | R2 = 0.97 η = 0.41 ± 0.003 | R2 = 0.98 η = 0.25 ± 002 d | 7.15 ± 0.34 a | 1530.3 ± 35.6 d |
| CSM–Blg–Ca3 | R2 = 0.92 η = 2.60 ± 0.30 | R2 = 0.93 η = 2.27 ± 0.36 | R2 = 0.94 η = 0.44 ± 0.004 | R2 = 0.95 η = 0.28 ± 0.001 c | 7.33 ± 0.28 ab | 1648.2 ± 41.4 c |
| CSM–Blg–Na1 | R2 = 0.87 η = 1.76 ± 0.24 | R2= 0.90 η = 1.60 ± 0.11 | R2 = 0.93 η = 0.31 ± 0.004 | R2 = 0.98 η = 0.20 ± 002 c | 6.64 ± 0.41 b | 1225.6 ± 24.9 c |
| CSM–Blg–Na2 | R2= 0.74 η = 1.18 ± 0.13 | R2 = 0.80 η = 1.25 ± 0.08 | R2 = 0.94 η = 0.40 ± 0.004 | R2 = 0.95 η = 0.22 ± 0.001 b | 6.85 ± 0.21 b | 1345.2 ± 20.1 b |
| CSM–Blg–Na3 | R2 = 0.92 η = 1.36 ± 0.11 | R2 = 0.93 η = 1.56 ± 0.13 | R2 = 0.98 η = 0.35 ± 0.004 | R2 = 0.98 η = 0.18 ± 0.001 d | 6.41 ± 0.16 b | 1102.2 ± 14.8 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheri, A.; Kashaninejad, M.; Tamaddon, A.M.; Du, J.; Jafari, S.M. Rheological Characteristics of Soluble Cress Seed Mucilage and β-Lactoglobulin Complexes with Salts Addition: Rheological Evidence of Structural Rearrangement. Gels 2023, 9, 485. https://doi.org/10.3390/gels9060485
Taheri A, Kashaninejad M, Tamaddon AM, Du J, Jafari SM. Rheological Characteristics of Soluble Cress Seed Mucilage and β-Lactoglobulin Complexes with Salts Addition: Rheological Evidence of Structural Rearrangement. Gels. 2023; 9(6):485. https://doi.org/10.3390/gels9060485
Chicago/Turabian StyleTaheri, Afsaneh, Mahdi Kashaninejad, Ali Mohammad Tamaddon, Juan Du, and Seid Mahdi Jafari. 2023. "Rheological Characteristics of Soluble Cress Seed Mucilage and β-Lactoglobulin Complexes with Salts Addition: Rheological Evidence of Structural Rearrangement" Gels 9, no. 6: 485. https://doi.org/10.3390/gels9060485