Formulation and Characterization of Emulgel-Based Jelly Candy: A Preliminary Study on Nutraceutical Delivery
Abstract
:1. Introduction
2. Results and Discussion
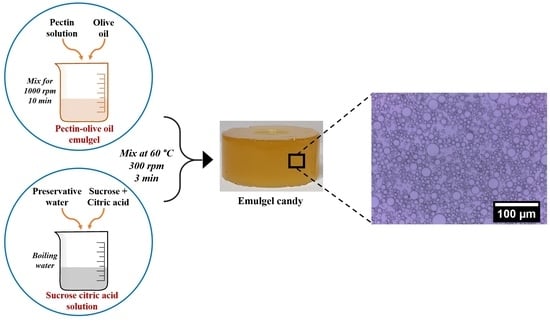
2.1. Preparation of Candy
2.2. Colorimetry Analysis
2.3. pH Analysis
2.4. FTIR Spectroscopy
2.5. DSC Analysis
2.6. Mechanical Analysis
2.7. In Vitro Disintegration Test
2.8. Drug Release Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methodology
Preparation of the Candy Formulations
4.3. Characterization of the Candies
4.3.1. pH Measurement
4.3.2. Colorimetry Analysis
4.3.3. FTIR Analysis
4.3.4. Disintegration Time Study
4.3.5. In Vitro Drug Release Study
4.3.6. Mechanical Analysis
4.3.7. DSC Analysis
4.3.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.-G.; Singh, A.P. Emerging strategies for enhancing buccal and sublingual administration of nutraceuticals and pharamaceuticals. J. Drug Deliv. Sci. Technol. 2019, 52, 440–451. [Google Scholar] [CrossRef]
- Shen, T.; Fan, S.; Li, Y.; Xu, G.; Fan, W. Preparation of Edible Non-wettable Coating with Soybean Wax for Repelling Liquid Foods with Little Residue. Materials 2020, 13, 3308. [Google Scholar] [CrossRef] [PubMed]
- Cappa, C.; Lavelli, V.; Mariotti, M. Fruit candies enriched with grape skin powders: Physicochemical properties. LWT-Food Sci. Technol. 2015, 62, 569–575. [Google Scholar] [CrossRef]
- Delgado, P.; Bañón, S. Effects of replacing starch by inulin on the physicochemical, texture and sensory characteristics of gummy jellies. CyTA-J. Food 2018, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Z.; Legrand, A.; Aoyama, T.; Ma, N.; Wang, W.; Otake, K.-I.; Urayama, K.; Horike, S.; Kitagawa, S. Hypercrosslinked Polymer Gels as a Synthetic Hybridization Platform for Designing Versatile Molecular Separators. J. Am. Chem. Soc. 2022, 144, 6861–6870. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Li, P.; Liu, F.-Q.; Guo, H.; Li, W.-H. Recent advances of organogels: From fabrications and functions to applications. POrCo 2021, 159, 106417. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. Preparation, structure-property relationships and applications of different emulsion gels: Bulk emulsion gels, emulsion gel particles, and fluid emulsion gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Xu, W.; Huang, L.; Jin, W.; Ge, P.; Shah, B.R.; Zhu, D.; Jing, J. Encapsulation and release behavior of curcumin based on nanoemulsions-filled alginate hydrogel beads. Int. J. Biol. Macromol. 2019, 134, 210–215. [Google Scholar] [CrossRef]
- de Souza Paglarini, C.; de Figueiredo Furtado, G.; Honório, A.R.; Mokarzel, L.; da Silva Vidal, V.A.; Ribeiro, A.P.B.; Cunha, R.L.; Pollonio, M.A.R. Functional emulsion gels as pork back fat replacers in Bologna sausage. Food Struct. 2019, 20, 100105. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Cui, M.; Yuan, F.; Gao, Y. Effect of the solid fat content on properties of emulsion gels and stability of β-carotene. J. Agric. Food Chem. 2019, 67, 6466–6475. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, S.R.; Karaca, A.C.; Deng, L.; Wang, Y.; Li, H.; Askari, G.; Rostamabadi, H. Insights into whey protein-based carriers for targeted delivery and controlled release of bioactive components. Food Hydrocoll. 2022, 133, 108002. [Google Scholar] [CrossRef]
- Light, K.; Karboune, S. Emulsion, hydrogel and emulgel systems and novel applications in cannabinoid delivery: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8199–8229. [Google Scholar] [CrossRef] [PubMed]
- Ellinfeb, A. Vitamins Gone Gummy. N. Y. Times Feb. 2017, 28, 2017. [Google Scholar]
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U. Pectin-water interactions in foods–From powder to gel. Food Hydrocoll. 2018, 78, 109–119. [Google Scholar] [CrossRef]
- Moslemi, M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr. Polym. 2021, 254, 117324. [Google Scholar] [CrossRef]
- Wan, L.; Wang, H.; Zhu, Y.; Pan, S.; Cai, R.; Liu, F.; Pan, S. Comparative study on gelling properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, atmospheric enzymatic, and alkaline de-esterification. Carbohydr. Polym. 2019, 226, 115285. [Google Scholar] [CrossRef]
- Cai, R.; Pan, S.; Li, R.; Xu, X.; Pan, S.; Liu, F. Curcumin loading and colon release of pectin gel beads: Effect of different de-esterification method. Food Chem. 2022, 389, 133130. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Souki, N.P.; Moraes, I.C.; Pinho, S.C. Rheology of emulsion-filled gels applied to the development of food materials. Gels 2016, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, S.; Oliver, C.M.; Ajlouni, S.; Augustin, M.A. Physicochemical characterisation and oxidative stability of fish oil and fish oil–extra virgin olive oil microencapsulated by sugar beet pectin. Food Chem. 2011, 127, 1694–1705. [Google Scholar] [CrossRef]
- Stanos, S. Topical analgesics. Phys. Med. Rehabil. Clin. 2020, 31, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Badola, A.; Goyal, M.; Baluni, S. Gels and Jellies a Recent Technology in Semisolids: A Review. World J. Pharm. Res. 2021, 10, 461–475. [Google Scholar]
- Gad, H.A.; Roberts, A.; Hamzi, S.H.; Gad, H.A.; Touiss, I.; Altyar, A.E.; Kensara, O.A.; Ashour, M.L. Jojoba Oil: An updated comprehensive review on chemistry, pharmaceutical uses, and toxicity. Polymers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade Pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, K.; Descallar, F.B.A.; Li, A.; Yang, X.; Yang, H. Gelation behaviors of some special plant-sourced pectins: A review inspired by examples from traditional gel foods in China. Trends Food Sci. Technol. 2022, 126, 26–40. [Google Scholar] [CrossRef]
- Wang, H.; Wan, L.; Chen, D.; Guo, X.; Liu, F.; Pan, S. Unexpected gelation behavior of citrus pectin induced by monovalent cations under alkaline conditions. Carbohydr. Polym. 2019, 212, 51–58. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Emulsification properties of pectin. Pectin Technol. Physiol. Prop. 2020, 83–97. [Google Scholar]
- Pan, L.-H.; Wu, C.-L.; Luo, S.-Z.; Luo, J.-P.; Zheng, Z.; Jiang, S.-T.; Zhao, Y.-Y.; Zhong, X.-Y. Preparation and characteristics of sucrose-resistant emulsions and their application in soft candies with low sugar and high lutein contents and strong antioxidant activity. Food Hydrocoll. 2022, 129, 107619. [Google Scholar] [CrossRef]
- Alam, S.S.; Bharti, D.; Pradhan, B.K.; Sahu, D.; Dhal, S.; Kim, N.M.; Jarzębski, M.; Pal, K. Analysis of the Physical and Structure Characteristics of Reformulated Pizza Bread. Foods 2022, 11, 1979. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, V.; Muste, S. Application of analytical methods for the comprehensive analysis of oleogels—A review. Polymers 2021, 13, 1934. [Google Scholar] [CrossRef]
- Becerra-Herrera, M.; Vélez-Martín, A.; Ramos-Merchante, A.; Richter, P.; Beltrán, R.; Sayago, A. Characterization and evaluation of phenolic profiles and color as potential discriminating features among Spanish extra virgin olive oils with protected designation of origin. Food Chem. 2018, 241, 328–337. [Google Scholar] [CrossRef]
- De Lima, A.C.V.; Dionisio, A.P.; de Abreu, F.A.P.; da Silva, G.S.; Junior, R.D.L.; Magalhães, H.C.R.; dos Santos Garruti, D.; da Silva Araújo, I.M.; Artur, A.G.; Taniguchi, C.A.K. Microfiltered red–purple pitaya colorant: UPLC-ESI-QTOF-MSE-based metabolic profile and its potential application as a natural food ingredient. Food Chem. 2020, 330, 127222. [Google Scholar] [CrossRef]
- Evageliou, V.; Richardson, R.; Morris, E. Effect of pH, sugar type and thermal annealing on high-methoxy pectin gels. Carbohydr. Polym. 2000, 42, 245–259. [Google Scholar] [CrossRef]
- Patil, N.V.; Netravali, A.N. Enhancing strength of wool fiber using a soy flour sugar-based “green” cross-linker. ACS Omega 2019, 4, 5392–5401. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Kamel, S. Development of graphene oxide-based styrene/acrylic elastomeric disks from sugarcane bagasse as adsorbents of Nickel (II) ions. J. Polym. Res. 2022, 29, 75. [Google Scholar] [CrossRef]
- Romano, N.; Santos, M.; Mobili, P.; Vega, R.; Gómez-Zavaglia, A. Effect of sucrose concentration on the composition of enzymatically synthesized short-chain fructo-oligosaccharides as determined by FTIR and multivariate analysis. Food Chem. 2016, 202, 467–475. [Google Scholar] [CrossRef]
- Brizuela, A.B.; Castillo, M.V.; Raschi, A.B.; Davies, L.; Romano, E.; Brandán, S.A. A complete assignment of the vibrational spectra of sucrose in aqueous medium based on the SQM methodology and SCRF calculations. Carbohydr. Res. 2014, 388, 112–124. [Google Scholar] [CrossRef]
- Bichara, L.C.; Lanús, H.E.; Brandán, S.A. Stabilities of aqueous solutions of sucrose containing ascorbic and citric acids by using FTIR spectroscopy and physicochemical studies. J. Mol. Liq. 2014, 200, 448–459. [Google Scholar] [CrossRef]
- Santos, E.E.; Amaro, R.C.; Bustamante, C.C.C.; Guerra, M.H.A.; Soares, L.C.; Froes, R.E.S. Extraction of pectin from agroindustrial residue with an ecofriendly solvent: Use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification. Food Hydrocoll. 2020, 107, 105921. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Baum, A.; Dominiak, M.; Vidal-Melgosa, S.; Willats, W.G.; Søndergaard, K.M.; Hansen, P.W.; Meyer, A.S.; Mikkelsen, J.D. Prediction of pectin yield and quality by FTIR and carbohydrate microarray analysis. Food Bioproc. Technol. 2017, 10, 143–154. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Siedle, I.; Boni, F.I.; Chorilli, M.; Müller, I.; Cury, B.S.F. Mucoadhesive films based on gellan gum/pectin blends as potential platform for buccal drug delivery. Pharm. Dev. Technol. 2020, 25, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Zhou, Y.; Yu, H.; Lu, C.; Han, K. Investigation on thermal degradation properties of oleic acid and its methyl and ethyl esters through TG-FTIR. Energy Convers. Manag. 2017, 149, 495–504. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B.; Tokatli, F. Use of FTIR and UV–visible spectroscopy in determination of chemical characteristics of olive oils. Talanta 2019, 201, 65–73. [Google Scholar] [CrossRef]
- De la Mata, P.; Dominguez-Vidal, A.; Bosque-Sendra, J.M.; Ruiz-Medina, A.; Cuadros-Rodríguez, L.; Ayora-Cañada, M.J. Olive oil assessment in edible oil blends by means of ATR-FTIR and chemometrics. Food Control 2012, 23, 449–455. [Google Scholar] [CrossRef]
- Rabelo, S.N.; Ferraz, V.P.; Oliveira, L.S.; Franca, A.S. FTIR analysis for quantification of fatty acid methyl esters in biodiesel produced by microwave-assisted transesterification. Int. J. Environ. Sci. Dev. 2015, 6, 964. [Google Scholar] [CrossRef] [Green Version]
- Tena Pajuelo, N.; Aparicio Ruiz, R.; García-González, D.L. Use of polar and nonpolar fractions as additional information sources for studying thermoxidized virgin olive oils by FTIR. Grasa Y Aceites 2014, 65. [Google Scholar]
- Singh, V.K.; Ramesh, S.; Pal, K.; Anis, A.; Pradhan, D.K.; Pramanik, K. Olive oil based novel thermo-reversible emulsion hydrogels for controlled delivery applications. J. Mater. Sci. Mater. Med. 2014, 25, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, H.; Le Dréau, Y.; Piccerelle, P.; Kister, J. The evaluation of cosmetic and pharmaceutical emulsions aging process using classical techniques and a new method: FTIR. Int. J. Pharm. 2005, 289, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Salehi, N.; Dehaghani, A.S.; Haghighi, M. Investigation of fluid-fluid interaction between surfactant-ion-tuned water and crude oil: A new insight into asphaltene behavior in the emulsion interface. J. Mol. Liq. 2023, 376, 121311. [Google Scholar] [CrossRef]
- Kubbutat, P.; Kulozik, U. Interactions of sugar alcohol, di-saccharides and polysaccharides with polysorbate 80 as surfactant in the stabilization of foams. Colloids Surf. Physicochem. Eng. Asp. 2021, 616, 126349. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Anwar, J.; Khan, S. Synthesis and characterization of biodegradable hydrogels for oral delivery of 5-fluorouracil targeted to colon: Screening with preliminary in vivo studies. Adv. Polym. Technol. 2018, 37, 221–229. [Google Scholar] [CrossRef]
- Hyang Aee, L.; Nam Hie, K.; Nishinari, K. DSC and rheological studies of the effects of sucrose on the gelatinization and retrogradation of acorn starch. Thermochim. Acta 1998, 322, 39–46. [Google Scholar] [CrossRef]
- Lee, J.W.; Thomas, L.C.; Schmidt, S.J. Can the thermodynamic melting temperature of sucrose, glucose, and fructose be measured using rapid-scanning differential scanning calorimetry (DSC)? J. Agric. Food Chem. 2011, 59, 3306–3310. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Khan, K.U.; Sohail, M.; Khalid, I. Functionalized pectin hydrogels by cross-linking with monomer: Synthesis, characterization, drug release and pectinase degradation studies. Polym. Bull. 2020, 77, 339–356. [Google Scholar]
- Queiroz, M.B.; Sousa, F.R.; da Silva, L.B.; Alves, R.M.V.; Alvim, I.D. Co-crystallized sucrose-soluble fiber matrix: Physicochemical and structural characterization. LWT 2022, 154, 112685. [Google Scholar] [CrossRef]
- Yadav, I.; Nayak, S.K.; Rathnam, V.S.; Banerjee, I.; Ray, S.S.; Anis, A.; Pal, K. Reinforcing effect of graphene oxide reinforcement on the properties of poly (vinyl alcohol) and carboxymethyl tamarind gum based phase-separated film. J. Mech. Behav. Biomed. Mater. 2018, 81, 61–71. [Google Scholar] [CrossRef]
- Satapathy, M.; Quereshi, D.; Nguyen, T.T.H.; Pani, D.; Mohanty, B.; Anis, A.; Maji, S.; Kim, D.; Sarkar, P.; Pal, K. Preparation and characterization of cocoa butter and whey protein isolate based emulgels for pharmaceutical and probiotics delivery applications. J. Dispers. Sci. Technol. 2019, 41, 426–440. [Google Scholar] [CrossRef]
- Raphael, A.P.; Crichton, M.L.; Falconer, R.J.; Meliga, S.; Chen, X.; Fernando, G.J.; Huang, H.; Kendall, M.A. Formulations for microprojection/microneedle vaccine delivery: Structure, strength and release profiles. J. Control. Release 2016, 225, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Filippo, E.; Serra, A.; Buccolieri, A.; Manno, D. Green synthesis of silver nanoparticles with sucrose and maltose: Morphological and structural characterization. J. Non-Cryst. Solids 2010, 356, 344–350. [Google Scholar] [CrossRef]
- Hosseini, A.; Bagheri, F.; Mohammadi, G.; Azami, M.; Tahvilian, R. Design and preparation of oral jelly candies of acetaminophen and its nanoparticles. Appl. Nanosci. 2022, 12, 101–107. [Google Scholar] [CrossRef]
- de Moura, S.C.; Berling, C.L.; Garcia, A.O.; Queiroz, M.B.; Alvim, I.D.; Hubinger, M.D. Release of anthocyanins from the hibiscus extract encapsulated by ionic gelation and application of microparticles in jelly candy. Food Res. Int. 2019, 121, 542–552. [Google Scholar] [CrossRef]
- Dewi, E.; Kurniasih, R.; Purnamayati, L. The application of microencapsulated phycocyanin as a blue natural colorant to the quality of jelly candy. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yogyakarta, Indonesia, 2–4 October 2018; p. 012047. [Google Scholar]
- Jain, A.; Pradhan, B.K.; Mahapatra, P.; Ray, S.S.; Chakravarty, S.; Pal, K. Development of a low-cost food color monitoring system. Color Res. Appl. 2021, 46, 430–445. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Calín-Sánchez, Á.; Clemente-Villalba, J.; Hernández, F.; Carbonell-Barrachina, Á.A.; Sendra, E.; Wojdyło, A. Quality parameters and consumer acceptance of jelly candies based on pomegranate juice “Mollar de Elche”. Foods 2020, 9, 516. [Google Scholar] [CrossRef] [Green Version]
- Thapliyal, S.; Bhatt, G.; Kandpal, G. Orodispersible Tablets: A Review. World J. Pharm. Res 2018, 7, 146–162. [Google Scholar]
- Qureshi, D.; Behera, K.P.; Mohanty, D.; Mahapatra, S.K.; Verma, S.; Sukyai, P.; Banerjee, I.; Pal, S.K.; Mohanty, B.; Kim, D. Synthesis of novel poly (vinyl alcohol)/tamarind gum/bentonite-based composite films for drug delivery applications. Colloids Surf. Physicochem. Eng. Asp. 2021, 613, 126043. [Google Scholar] [CrossRef]
- Qureshi, D.; Nadikoppula, A.; Mohanty, B.; Anis, A.; Cerqueira, M.; Varshney, M.; Pal, K. Effect of carboxylated carbon nanotubes on physicochemical and drug release properties of oleogels. Colloids Surf. Physicochem. Eng. Asp. 2021, 610, 125695. [Google Scholar] [CrossRef]
- Yu, H.; Yu, J.; Zhan, M. Study on mechanical behaviour of agar gel in compression mode. Bulg. Chem. Commun. 2018, 1, 225. [Google Scholar]











| S. No. | Sample Name | SCS (g) | Emulsion | Nutraceutical Agents | ||
|---|---|---|---|---|---|---|
| PS (g) | Olive Oil (g) | Curcumin (mg) | Riboflavin (mg) | |||
| 1 | AP00 | 35 | 15 | -- | -- | -- |
| 2 | AP10 | 35 | 13.5 | 1.5 | -- | -- |
| 3 | AP15 | 35 | 12.75 | 2.25 | -- | -- |
| 4 | AP20 | 35 | 12 | 3 | -- | -- |
| 5 | AP25 | 35 | 11.25 | 3.75 | -- | -- |
| 6 | AP30 | 35 | 10.5 | 4.5 | -- | -- |
| 7 | AP00-C | 35 | 15 | -- | 150 | -- |
| 8 | AP10-C | 35 | 13.5 | 1.5 | 150 | -- |
| 9 | AP15-C | 35 | 12.75 | 2.25 | 150 | -- |
| 10 | AP20-C | 35 | 12 | 3 | 150 | -- |
| 11 | AP25-C | 35 | 11.25 | 3.75 | 150 | -- |
| 12 | AP30-C | 35 | 10.5 | 4.5 | 150 | -- |
| 13 | AP00-R | 35 | 15 | -- | -- | 250 |
| 14 | AP10-R | 35 | 13.5 | 1.5 | -- | 250 |
| 15 | AP15-R | 35 | 12.75 | 2.25 | -- | 250 |
| 16 | AP20-R | 35 | 12 | 3 | -- | 250 |
| 17 | AP25-R | 35 | 11.25 | 3.75 | -- | 250 |
| 18 | AP30-R | 35 | 10.5 | 4.5 | -- | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhal, S.; Pal, A.; Gramza-Michalowska, A.; Kim, D.; Mohanty, B.; Sagiri, S.S.; Pal, K. Formulation and Characterization of Emulgel-Based Jelly Candy: A Preliminary Study on Nutraceutical Delivery. Gels 2023, 9, 466. https://doi.org/10.3390/gels9060466
Dhal S, Pal A, Gramza-Michalowska A, Kim D, Mohanty B, Sagiri SS, Pal K. Formulation and Characterization of Emulgel-Based Jelly Candy: A Preliminary Study on Nutraceutical Delivery. Gels. 2023; 9(6):466. https://doi.org/10.3390/gels9060466
Chicago/Turabian StyleDhal, Somali, Anupam Pal, Anna Gramza-Michalowska, Doman Kim, Biswaranjan Mohanty, Sai S. Sagiri, and Kunal Pal. 2023. "Formulation and Characterization of Emulgel-Based Jelly Candy: A Preliminary Study on Nutraceutical Delivery" Gels 9, no. 6: 466. https://doi.org/10.3390/gels9060466