Green Synthesis of Hydrogel-Based Adsorbent Material for the Effective Removal of Diclofenac Sodium from Wastewater
Abstract
:1. Introduction
2. Results and Discussion
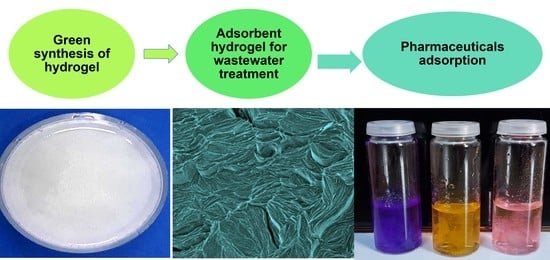
2.1. Visual Examination
2.2. Infrared Spectroscopy Measurements
2.3. Raman Spectroscopy Results
2.4. XRD Results
2.5. Differential Scanning Calorimetry (DSC) Analysis
2.6. Thermogravimetric and Differential Thermal Gravimetric Analysis
2.7. SEM Analysis
2.8. Rheology
2.9. Pharmacotehnical Characterization
2.10. Preliminary Adsorption Studies
2.11. Batch Adsorption Study
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. CPX Hydrogel Preparation
4.3. Methods
4.3.1. Visual Examination
4.3.2. Physical and Chemical Analysis
4.3.3. Pharmacotechnical Characterization and Elongation Ability and Tensile Strength
Moisture Content
Swelling Behavior
4.4. Preliminary Adsorption Studies
4.5. Batch Adsorption Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du Plessis, A. Persistent degradation: Global water quality challenges and required actions. One Earth 2022, 5, 129–131. [Google Scholar] [CrossRef]
- Estêvão, M.D. Aquatic Pollutants: Risks, Consequences, Possible Solutions and Novel Testing Approaches. Fishes 2023, 8, 97. [Google Scholar] [CrossRef]
- Dulsat-Masvidal, M.; Ciudad, C.; Infante, O.; Mateo, R.; Lacorte, S. Water pollution threats in important bird and biodiversity areas from Spain. J. Hazard. Mater. 2023, 448, 130938. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, A.; Ahmaruzzaman, M. Recent advances in new generation nanocomposite materials for adsorption of pharmaceuticals from aqueous environment. Environ. Sci. Pollut. Res. 2023, 30, 39377–39417. [Google Scholar] [CrossRef]
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current research trends on emerging contaminants pharmaceutical and personal care products (PPCPs): A comprehensive review. Sci. Total Environ. 2023, 859, 160031. [Google Scholar] [CrossRef]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of diclofenac from wastewater: A comprehensive review of detection, characteristics and tertiary treatment techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Matamoros, V.; Rodríguez, Y.; Albaig’es, J. A comparative assessment of intensive and extensive wastewater treatment technologies for removing emerging contaminants in small communities. Water Res. 2016, 88, 777–785. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Cuprys, A.K.; Maletskyi, Z.; Ratnaweera, H. Treatment Trends and Combined Methods in Removing Pharmaceuticals and Personal Care Products from Wastewater—A Review. Membranes 2023, 13, 158. [Google Scholar] [CrossRef]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes 2023, 13, 181. [Google Scholar] [CrossRef]
- Rikabi, A.A.K.K.; Chelu, B.; Mariana; Harabor, I.; Albu, P.C.; Segarceanu, M.; Nechifor, G. Iono-molecular Separation with Composite Membranes I. Preparation and characterization of membranes with polysulfone matrix. Rev. Chimie 2016, 67, 1658–1665. [Google Scholar]
- Diaconu, I.; Gardea, R.; Cristea, C.; Nechifor, G.; Ruse, E.; Eftimie Totu, E. Removal and recovery of some phenolic pollutants using liquid membranes. Rom. Biotechnol. Lett. 2010, 15, 2010. [Google Scholar]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Pîrtac, A.; Albu, P.C.; Oprea, O.; Grosu, A.R.; Pascu, D.; Pancescu, F.M.; Nechifor, G.; et al. Reactional Processes on Osmium–Polymeric Membranes for 5–Nitrobenzimidazole Reduction. Membranes 2021, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, I.; Aboul-Enein, H.Y.; Bunaciu, A.A.; Ruse, E.; Mirea, C.; Nechifor, G. Selective separation of acetaminophene from pharmaceutical formulations through membrane techniques. Rev. Roum. Chim. 2015, 60, 521–525. [Google Scholar]
- Serban, E.A.; Diaconu, I.; Ruse, E.; Ghe, B.; Nechifor, G.; Lazar, M.N. Bulk Liquid Membranes for Separation and Recovery of Pharmaceutical Products. Rev. Chim. 2018, 69, 3257–3260. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Bhatt, P.; Joshi, S.; Urper Bayram, G.M.; Khati, P.; Simsek, H. Developments and application of chitosan-based adsorbents for wastewater treatments. Environ. Res. 2023, 226, 115530. [Google Scholar] [CrossRef]
- Bezerra de Araujo, C.M.; Ghislandi, M.G.; Gonçalves Rios, A.; Bezerra da Costa, G.R.; do Nascimento, B.F.; Ferreira, A.F.P.; da Motta Sobrinho, M.A.; Rodrigues, A.E. Wastewater treatment using recyclable agar-graphene oxide biocomposite hydrogel in batch and fixed-bed adsorption column: Bench experiments and modeling for the selective removal of organics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128357. [Google Scholar] [CrossRef]
- Resende, J.F.; Paulino, I.M.R.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Hydrogels produced from natural polymers: A review on its use and employment in water treatment. Braz. J. Chem. Eng. 2023, 40, 23–38. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef]
- Chelu, M.; Popa, M.; Ozon, E.A.; Pandele Cusu, J.; Anastasescu, M.; Surdu, V.A.; Calderon Moreno, J.; Musuc, A.M. High-Content Aloe vera Based Hydrogels: Physicochemical and Pharmaceutical Properties. Polymers 2023, 15, 1312. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef]
- Khan, S.A.; Shah, L.A.; Shah, M.; Jamil, I. Engineering of 3D polymer network hydrogels for biomedical applications: A review. Polym. Bull. 2022, 79, 2685–2705. [Google Scholar] [CrossRef]
- Bezerra de Araujo, C.M.; Wernke, G.; Ghislandi, M.G.; Diório, A.; Vieira, M.F.; Bergamasco, R.; da Motta Sobrinho, M.A.; Rodrigues, A.E. Continuous removal of pharmaceutical drug chloroquine and Safranin-O dye from water using agar-graphene oxide hydrogel: Selective adsorption in batch and fixed-bed experiments. Environ. Res. 2023, 216, 114425. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.S.; Crestani, L.; Marchezi, G.; Melara, F.; Rafael de Mello, J.; Dotto, G.L.; Piccin, J.S. Synthesis of glutaraldehyde-modified silica/chitosan composites for the removal of water-soluble diclofenac sodium. Carbohydr. Polym. 2022, 277, 118868. [Google Scholar] [CrossRef]
- Mottaghi, H.; Mohammadi, Z.; Abbasi, M.; Tahouni, N.; Panjeshahi, M.H. Experimental investigation of crude oil removal from water using polymer adsorbent. J. Water Process Eng. 2021, 40, 101959. [Google Scholar] [CrossRef]
- Gkika, D.A.; Mitropoulos, A.C.; Kokkinos, P.; Lambropoulou, D.A.; Kalavrouziotis, I.K.; Bikiaris, D.N.; Kyzas, G.Z. Modified chitosan adsorbents in pharmaceutical simulated wastewaters: A review of the last updates. Carbohydr. Polym. Technol. Appl. 2023, 5, 100313. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Deng, S.; Wang, R.; Xu, H.; Jiang, X.; Yin, J. Hybrid hydrogels of hyperbranched poly(ether amine)s (hPEAs) for selective adsorption of guest molecules and separation of dyes. J. Mater. Chem. 2012, 22, 10055–10061. [Google Scholar] [CrossRef]
- Zare, E.N.; Fallah, Z.; Le, V.T.; Doan, V.-D.; Mudhoo, A.; Joo, S.-W.; Vasseghian, Y.; Tajbakhsh, M.; Moradi, O.; Sillanpaa, M.; et al. Remediation of pharmaceuticals from contaminated water by molecularly imprinted polymers: A review. Environ. Chem. Lett. 2022, 20, 2629–2664. [Google Scholar] [CrossRef]
- Fortunato, A.; Mba, M. A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation. Gels 2022, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Samanta, R.; Ahmed, S.; Pramanik, B. Disulphide Cross-Linked Ultrashort Peptide Hydrogelator for Water Remediation. Chem. Eur. J. 2023, e202300312. [Google Scholar] [CrossRef]
- Grohs, L.; Cheng, L.; Cönen, S.; Haddad, B.G.; Bülow, A.; Toklucu, I.; Ernst, L.; Körner, J.; Schmalzing, G.; Lampert, A.; et al. Diclofenac and other non-steroidal anti-inflammatory drugs (NSAIDs) are competitive antagonists of the human P2X3 receptor. Front. Pharmacol. 2023, 14, 1120360. [Google Scholar] [CrossRef] [PubMed]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in the marine environment: A review of its occurrence and effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Filho, J.A.A.; da Cruz, H.M.; Fernandes, B.S.; Motteran, F.; de Paiva, A.L.R.; da Silva Pereira Cabral, J.J. Efficiency of the bank filtration technique for diclofenac removal: A review. Environ. Pollut. 2022, 300, 118916. [Google Scholar] [CrossRef]
- Fahimi, A.; Zanoletti, A.; Federici, S.; Assi, A.; Bilo, F.; Depero, L.E.; Bontempi, E. New eco-materials derived from waste for emerging pollutants adsorption: The case of diclofenac. Materials 2020, 13, 3964. [Google Scholar] [CrossRef]
- Godiya, C.B.; Kumar, S.; Xiao, Y. Amine functionalized egg albumin hydrogel with enhanced adsorption potential for diclofenac sodium in water. J. Hazard. Mater. 2020, 393, 122417. [Google Scholar] [CrossRef]
- Khan, S.A.; Siddiqui, M.F.; Khan, T.A. Synthesis of Poly(methacrylic acid)/Montmorillonite Hydrogel Nanocomposite for Efficient Adsorption of Amoxicillin and Diclofenac from Aqueous Environment: Kinetic, Isotherm, Reusability, and Thermodynamic Investigations. ACS Omega 2020, 5, 2843–2855. [Google Scholar] [CrossRef]
- Feng, Z.; Simeone, A.; Odelius, K.; Hakkarainen, M. Biobased Nanographene Oxide Creates Stronger Chitosan Hydrogels with Improved Adsorption Capacity for Trace Pharmaceuticals. ACS Sustain. Chem. Eng. 2017, 5, 11525–11535. [Google Scholar] [CrossRef]
- Umbreen, N.; Sohni, S.; Ahmad, I.; Khattak, N.U.; Gul, K. Self-assembled three-dimensional reduced graphene oxide-based hydrogel for highly efficient and facile removal of pharmaceutical compounds from aqueous solution. J. Colloid Interface Sci. 2018, 527, 356–367. [Google Scholar] [CrossRef]
- Mahmoodi, H.; Fattahi, M.; Motevassel, M. Graphene oxide–chitosan hydrogel for adsorptive removal of diclofenac from aqueous solution: Preparation, characterization, kinetic and thermodynamic modelling. RSC Adv. 2021, 11, 36289–36304. [Google Scholar] [CrossRef] [PubMed]
- Zaka, A.; Ibrahim, T.H.; Khamis, M. Removal of selected non-steroidal anti-inflammatory drugs from wastewater using reduced graphene oxide magnetite. Desalination Water Treat. 2021, 212, 401–414. [Google Scholar] [CrossRef]
- Argin-Soysal, S.; Kofinas, P.; Lo, Y.M. Effect of complexation conditions on xanthan–chitosan polyelectrolyte complex gels. Food Hydrocoll. 2009, 23, 202–209. [Google Scholar] [CrossRef]
- Corrias, F.; Dolz, M.; Herraez, M.; Diez-Sales, O. Rheological properties of progesterone microemulsions: Influence of xanthan and chitosan biopolymer concentration. J. Appl. Polym. Sci. 2008, 110, 1225–1235. [Google Scholar] [CrossRef]
- Phaechamud, T.; Ritthidej, G.C. Formulation variables influencing drug release from layered matrix system comprising chitosan and xanthan gum. AAPS PharmSciTech 2008, 9, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Popa, N.; Novac, O.; Profire, L.; Lupusoru, C.E.; Popa, M.I. Hydrogels based on chitosan–xanthan for controlled release of theophylline. J. Mater. Sci. Mater. Med. 2010, 21, 1241–1248. [Google Scholar] [CrossRef]
- Bellini, M.Z.; Pires, A.L.R.; Vasconcelos, M.O.; Moraes, A.M. Comparison of the properties of compacted and porous lamellar chitosan–xanthan membranes as dressings and scaffolds for the treatment of skin lesions. J. Appl. Polym. Sci. 2012, 125, 421–431. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for a drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Chelu, M.; Moreno, J.C.; Atkinson, I.; Cusu, J.P.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.-M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel functional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef]
- Zajac, A.; Hanuza, J.; Wandas, M.; Dyminska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/Xanthan Gum Based Hydrogels as Potential Carrier for an Antiviral Drug: Fabrication, Characterization, and Safety Evaluation. Front Chem. 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Dey, S.C.; Al-Amin, M.; Rashid, T.U.; Sultan, M.Z.; Ashaduzzaman, M.; Sarker, M.; Shamsuddin, S.M. Reparation, characterization and performance evaluation of chitosan as an adsorbent for remazol red. Int. J. Latest Res. Eng. Technol. 2016, 2, 52–62. [Google Scholar]
- De Morais Lima, M.; Carneiro, L.C.; Bianchini, D.; Dias, R.G.A.; da Rosa Zavareze, E.; Prentice, C.; da Silveira Moreira, A. Structural, Thermal, Physical, Mechanical and Barrier Properties of Chitosan Films with the Addition of Xanthan Gum. J. Food Sci. 2017, 82, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.M.; Martins, V.C.A.; de Guzzi Plepis, A.M. Influence of collagen addition on the thermal and morphological properties of chitosan/xanthan hydrogels. Int. J. Biol. Macromol. 2015, 80, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, R.; Il, B.W.; Navamathavan, R.; El-Newehy, M.H.; Kim, H.Y. Preparation and characterizations of anisotropic chitosan nanofibers via electrospinning. Macromol. Res. 2011, 19, 345–350. [Google Scholar] [CrossRef]
- Hussien, M.A.; Ebtessam, A.E.; El, G.S.A. Investigation of the effect of formulation additives on telmisartan dissolution rate: Development of oral disintegrating tablets. Eur. J. Biomed. Pharm. Sci. 2019, 6, 12–20. [Google Scholar]
- Essa, E.; Elmarakby, A.; Donia, A.; El Maghraby, G.M. Controlled precipitation for enhanced dissolution rate of flurbiprofen: Development of rapidly disintegrating tablets. Drug Dev. Ind. Pharm. 2017, 24, 1–10. [Google Scholar] [CrossRef]
- Kuzmin, V.V.; Novikov, V.S.; Ustynyuk, L.Y.; Prokhorov, K.A.; Sagitova, E.A.; Nikolaeva, G.Y. Raman spectra of polyethylene glycols: Comparative experimental and DFT study. J. Mol. Struct. 2020, 1217, 128331. [Google Scholar] [CrossRef]
- Matsuura, H.; Fukuhara, K. Conformational analysis of poly(oxyethylene) chain in aqueous solution as a hydrophilic moiety of nonionic surfactants. J. Mol. Struct. 1985, 126, 251–260. [Google Scholar] [CrossRef]
- Matsuura, H.; Fukuhara, K. Vibrational spectroscopic studies of conformation of poly(oxyethylene). II. Conformation–spectrum correlations. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 1383–1400. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tadokoro, H. Structural studies of polyethers, (-(CH2)m-O-)n. X. crystal structure of poly(ethylene oxide). Macromolecules 1973, 6, 672–675. [Google Scholar] [CrossRef]
- Kumar, S.; Dutta, P.K.; Koh, J. A physiocochemical and biological study of novel chitosan-chloroquinoline derivative for biomedical applications. Int. J. Biol. Macromol. 2011, 49, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Podgorbunskikh, E.; Kuskov, T.; Rychkov, D.; Lomovskii, O.; Bychkov, A. Mechanical Amorphization of Chitosan with Different Molecular Weights. Polymers 2022, 14, 4438. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, X.; Jiang, L.; Wu, B.; Wang, J.; Lei, J. Solvent-free synthesis and properties of novel solid–solid phase change materials with biodegradable castor oil for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 147, 177–184. [Google Scholar] [CrossRef]
- Fița, A.C.; Secăreanu, A.A.; Musuc, A.M.; Ozon, E.A.; Sarbu, I.; Atkinson, I.; Rusu, A.; Mati, E.; Anuta, V.; Pop, A.L. The Influence of the Polymer Type on the Quality of Newly Developed Oral Immediate-Release Tablets Containing Amiodarone Solid Dispersions Obtained by Hot-Melt Extrusion. Molecules 2022, 27, 6600. [Google Scholar] [CrossRef]
- Kang, Y.; Li, P.; Zeng, X.; Chen, X.; Xie, Y.; Zeng, Y.; Zhang, Y.; Xie, T. Biosynthesis, structure and antioxidant activities of xanthan gum from Xanthomonas campestris with additional furfural. Carbohydr. Polym. 2019, 216, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.K.; Wan, K.P.; Yu, P.H. Miscibility and morphology of chiral semicrystalline poly-(R)-(3-hydroxybutyrate)/chitosan and poly-(R)-(3-hydroxybutyrate-co-3-hydroxyvalerate)/chitosan blends studied with DSC,1HT1 andT1? CRAMPS. J. Appl. Polym. Sci. 2002, 86, 1253–1258. [Google Scholar] [CrossRef]
- Kittur, F.; Prashanth, K.H.; Sankar, K.U.; Tharanathan, R. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Cardenas, G.; Miranda, S.P. FTIR and TGA studies of chitosan composite films. J. Chil. Chem. Soc. 2004, 49, 291–295. [Google Scholar] [CrossRef]
- Kacurakova, M.; Belton, P.S.; Hirsch, J.; Ebringerova, A. Hydration Properties of Xylan-Type Structures: An Study of Xylo-oligosaccharides FTIR. J. Sci. Food Agric. 1998, 77, 38–44. [Google Scholar] [CrossRef]
- Phillips, G.O.; Takigami, S.; Takigami, M. Hydration characteristics of the gum exudate from Acacia Senegal. Food Hydrocoll. 1996, 10, 11–19. [Google Scholar] [CrossRef]
- Appelquist, I.A.M.; Cooke, D.; Gidley, M.J.; Lane, S.J. Effect of Heat Treatment on the Pectins of Tomatoes during Tomato Paste Manufacturing. Carbohydr. Polym. 1993, 20, 291–299. [Google Scholar] [CrossRef]
- Gidley, M.J.; Robinson, G. 18—Techniques for Studying Interactions Between Polysaccharides. Methods Plant Biochem. 1990, 2, 607–642. [Google Scholar] [CrossRef]
- Sreenivasan, K. Thermal stability studies of some chitosan metal ion complexes using differential scanning calorimetry. Polym. Degrad. Stab. 1996, 52, 85–87. [Google Scholar] [CrossRef]
- Deng, L.; Qi, H.; Yao, C.; Feng, M.; Dong, A. Investigation on the properties of methoxy poly (ethylene glycol)/chitosan graft co-polymers. J. Biomater. Sci. Polym. Ed. 2007, 18, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.M.; Menezes, P.F.; Alves, R.M.; Gonçalves, R.J.; Junges, A.; Formentin, M.W.; Mendonça, D.F.; Ligabue, R.A.; Bueno, M.F.; Severino, P.; et al. Chitosan and chitosan/PEG nanoparticles loaded with in-dole-3-carbinol: Characterization, computational study and potential effect on human bladder cancer cells. Mater. Sci. Eng. C 2021, 124, 112089. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Reddy, G.V.S.; Reddy, A.B.; Sudhakar, K.; Sadiku, E.R. Preparation and characterization of poly(ethylene glycol) stabilized nano silver particles by a mechanochemical assisted ball mill process. J. Appl. Polym. Sci. 2016, 133, 43027. [Google Scholar] [CrossRef]
- Yang, J.H.; Han, Y.S.; Park, M.; Park, T.; Hwang, S.J.; Choy, J.H. New inorganic-based drug delivery system of indole-3-acetic acid-layered metal hydroxide nanohybrids with controlled release rate. Chem. Mater. 2007, 19, 2679–2685. [Google Scholar] [CrossRef]
- Chitra, G.; Franklin, D.S.; Guhanathan, S. Indole-3-acetic acid based tunable hydrogels for antibacterial, antifungal and antioxidant applications. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 151–163. [Google Scholar] [CrossRef]
- Yongmei, G.; Chengqun, Y.; Zhenzhong, Z.; Xinhao, W.; Abid, N.; Rui, Z.; Weifeng, Z. Chitosan/xanthan gum-based (Hydroxypropyl methylcellulose-co-2-Acrylamido-2-methylpropane sulfonic acid) interpenetrating hydrogels for con-trolled release of amorphous solid dispersion of bioactive constituents of Pueraria lobatae. Int. J. Biol. Macromol. 2023, 224, 380–395. [Google Scholar] [CrossRef]
- Kulkarni, N.; Wakte, P.; Naik, J. Development of floating chitosan-xanthan beads for oral controlled release of glipizide. Int. J. Pharm. Investig. 2015, 5, 73–80. [Google Scholar] [CrossRef]
- Nafisa, G.; Shahzad, M.K.; Osama, M.B.; Atif, I.; Attaullah, S.; Sehrish, J.; Saba, U.K.; Afrasyab, K.; Rafi, U.K.; Muhammad, T.Z.B. Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef]
- Lessa, E.F.; Nunes, M.L.; Fajardo, A.R. Chitosan/waste coffee-grounds composite: An efficient and eco-friendly adsorbent for removal of pharmaceutical contaminants from water. Carbohydr. Polym. 2018, 189, 257–266. [Google Scholar] [CrossRef]
- Martinez-Ruvalcaba, A.; Chornet, E.; Rodrigue, D. Viscoelastic properties of dispersed chitosan/xanthan hydrogels. Carbohydr. Polym. 2007, 67, 586–595. [Google Scholar] [CrossRef]
- Hu, D.; Huang, H.; Jiang, R.; Wang, N.; Xu, H.; Wang, Y.G.; Ouyang, X.K. Adsorption of diclofenac sodium on bilayer amino-functionalized cellulose nanocrystals/chitosan composite. J. Hazard. Mater. 2019, 369, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.X.; Omer, A.M.; Hu, Z.-H.; Wang, Y.G.; Di, Y.; Ouyang, X.-K. Efficient adsorption of diclofenac sodium from aqueous solutions using magnetic amine-functionalized chitosan. Chemosphere 2019, 217, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cui, J.; Wu, X.; Zhang, X.; Hu, Q.; Hou, X. Rapid in situ microwave synthesis of Fe3O4@MIL-100(Fe) for aqueous diclofenac sodium removal through integrated adsorption and photodegradation. J. Hazard. Mater. 2019, 373, 408–416. [Google Scholar] [CrossRef]
- Zhuang, S.; Cheng, R.; Wang, J. Adsorption of diclofenac from aqueous solution using UiO-66-type metal-organic frameworks. Chem. Eng. J. 2019, 359, 354–362. [Google Scholar] [CrossRef]
- Xiong, T.; Yuan, X.; Wang, H.; Wu, Z.; Jiang, L.; Leng, L.; Xi, K.; Cao, X.; Zeng, G. Highly efficient removal of diclofenac sodium from medical wastewater by Mg/Al layered double hydroxide-poly(m-phenylenediamine) composite. Chem. Eng. J. 2019, 366, 83–91. [Google Scholar] [CrossRef]
- Zhao, R.; Zheng, H.; Zhong, Z.; Zhao, C.; Sun, Y.; Huang, Y.; Zheng, X. Efficient removal of diclofenac from surface water by the functionalized multilayer magnetic adsorbent: Kinetics and mechanism. Sci. Total Environ. 2021, 760, 144307. [Google Scholar] [CrossRef]
- Balaci, T.; Velescu, B.; Karampelas, O.; Musuc, A.M.; Nitulescu, G.M.; Ozon, E.A.; Nitulescu, G.; Gird, C.E.; Fita, C.; Lupuliasa, D. Physico-Chemical and Pharmaco-Technical Characterization of Inclusion Complexes Formed by Rutoside with beta-Cyclodextrin and Hydroxypropyl-beta-Cyclodextrin Used to Develop Solid Dosage Forms. Processes 2021, 9, 26. [Google Scholar] [CrossRef]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive buccal patches of miconazole nitrate: In vitro/in vivo performance and effect of ageing. Int. J. Pharm. 2003, 264, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Don, T.M.; Huang, M.L.; Chiu, A.C. Preparation of thermo-responsive acrylic hydrogels useful for the application in transdermal drug delivery systems. Mater Chem Phys 2008, 107, 266–273. [Google Scholar] [CrossRef]
- Wang, K.; Fu, Q.; Chen, X.; Gao, Y.; Dong, K. Preparation and characterization of pH-sensitive hydrogel for drug delivery system. RSC Adv. 2012, 2, 7772–7780. [Google Scholar] [CrossRef]
- Yi, Z.; Yao, J.; Zhu, M.; Chen, H.; Wang, F.; Liu, X. Kinetics, equilibrium, and thermodynamics investigation on the adsorption of lead (II) by coal-based activated carbon. SpringerPlus 2016, 5, 1160. [Google Scholar] [CrossRef] [PubMed]















| Pseudo First-Order Parameters | [CPX] | ||||
|---|---|---|---|---|---|
| 10 mg | 25 mg | 50 mg | 100 mg | 200 mg | |
| k1/(min−1) | 0.018 | 0.012 | 0.013 | 0.004 | 0.006 |
| qe /(mg × g−1) | 208.67 | 202.44 | 189.93 | 187.06 | 175.01 |
| R2 | 0.99 | 0.99 | 0.98 | 0.98 | 0.99 |
| Langmuir Isotherm Parameters | Freundlich Isotherm Parameters | |||||
|---|---|---|---|---|---|---|
| qmax | b | RL | R2 | KF | n | R2 |
| 172.41 | 0.0014 | 0.80 | 0.99 | 292.33 | 16.28 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelu, M.; Popa, M.; Calderon Moreno, J.; Leonties, A.R.; Ozon, E.A.; Pandele Cusu, J.; Surdu, V.A.; Aricov, L.; Musuc, A.M. Green Synthesis of Hydrogel-Based Adsorbent Material for the Effective Removal of Diclofenac Sodium from Wastewater. Gels 2023, 9, 454. https://doi.org/10.3390/gels9060454
Chelu M, Popa M, Calderon Moreno J, Leonties AR, Ozon EA, Pandele Cusu J, Surdu VA, Aricov L, Musuc AM. Green Synthesis of Hydrogel-Based Adsorbent Material for the Effective Removal of Diclofenac Sodium from Wastewater. Gels. 2023; 9(6):454. https://doi.org/10.3390/gels9060454
Chicago/Turabian StyleChelu, Mariana, Monica Popa, Jose Calderon Moreno, Anca Ruxandra Leonties, Emma Adriana Ozon, Jeanina Pandele Cusu, Vasile Adrian Surdu, Ludmila Aricov, and Adina Magdalena Musuc. 2023. "Green Synthesis of Hydrogel-Based Adsorbent Material for the Effective Removal of Diclofenac Sodium from Wastewater" Gels 9, no. 6: 454. https://doi.org/10.3390/gels9060454