State-of-the-Art Insights and Potential Applications of Cellulose-Based Hydrogels in Food Packaging: Advances towards Sustainable Trends
Abstract
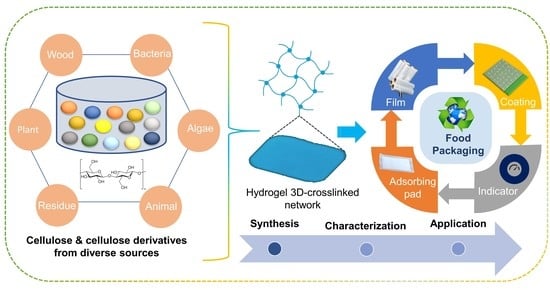
:1. Introduction
2. Hydrogel: Structural Chemistry and Classification
2.1. Structural Chemistry of Hydrogel
2.2. Classification of Hydrogel
3. Sustainable Hydrogels from Cellulose: Synthesis Routes and Characterization
3.1. Synthesis of Cellulose-Based Hydrogels (CBHs)
3.2. Characterization of CBHs
4. Application of CBHs in Food Packaging System
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jang, Y.; Kim, K.N.; Woo, J. Post-consumer plastic packaging waste from online food delivery services in South Korea. Waste Manag. 2023, 156, 177–186. [Google Scholar] [CrossRef]
- Korea, G. 2.1 Billion Packaging and Delivery Containers Were Produced; The Ministry of Environment Should Strengthen Regulations on the Use of Disposable Delivery Containers. 2021. Available online: https://www.greenkorea.org/activity/living-environment/zerowaste/90137/ (accessed on 28 April 2023).
- Environment Protection Agency (KMO). Establishing Measures to Reduce and Recycle the Plastic Generated in Life Cycles; Environment Protection Agency (KMO): Washington, DC, USA, 2020. [Google Scholar]
- Shin, S.-K.; Um, N.; Kim, Y.-J.; Cho, N.-H.; Jeon, T.-W. New Policy Framework with Plastic Waste Control Plan for Effective Plastic Waste Management. Sustainability 2020, 12, 6049. [Google Scholar] [CrossRef]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2022, 9, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased materials for food packaging. J. Bioresour. Bioprod. 2022, 7, 105–123. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Biodegradation of PVP–CMC hydrogel film: A useful food packaging material. Carbohydr. Polym. 2012, 89, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.D.S.S.; Freitas, M.M.; Cerqueira, M.A.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef]
- Koneru, A.; Dharmalingam, K.; Anandalakshmi, R. Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose–grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 2020, 148, 833–842. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels 2022, 9, 1. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Zafar, A.; Khosa, M.K.; Noor, A.; Qayyum, S.; Saif, M.J. Carboxymethyl Cellulose/Gelatin Hydrogel Films Loaded with Zinc Oxide Nanoparticles for Sustainable Food Packaging Applications. Polymers 2022, 14, 5201. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Tabibiazar, M.; Ghorbani, M.; Jahanban-Esfahlan, A. Fabrication and characterization of novel antibacterial chitosan/dialdehyde guar gum hydrogels containing pomegranate peel extract for active food packaging application. Int. J. Biol. Macromol. 2021, 187, 179–188. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Del Grosso, V.; Ferrari, G. Evaluation of the Physical Stability of Starch-Based Hydrogels Produced by High-Pressure Processing (HPP). Gels 2022, 8, 152. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2021, 30, 19–50. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, S.; Xia, X.; Tan, M.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Du, J.; Wang, H. High-performance carboxymethyl cellulose-based hydrogel film for food packaging and preservation system. Int. J. Biol. Macromol. 2022, 223, 1126–1137. [Google Scholar] [CrossRef]
- Pirayesh, H.; Park, B.-D.; Khanjanzadeh, H.; Park, H.-J.; Cho, Y.-J. Cellulosic material-based colorimetric films and hydrogels as food freshness indicators. Cellulose 2023, 30, 1–35. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Recent Advances in Cellulose-Based Hydrogels: Food Applications. Foods 2023, 12, 350. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, H.; Ma, L.; Zhou, H.; Yu, Y.; Guo, T.; Zhang, Y.; Huang, H. Green pH/magnetic sensitive hydrogels based on pineapple peel cellulose and polyvinyl alcohol: Synthesis, characterization and naringin prolonged release. Carbohydr. Polym. 2019, 209, 51–61. [Google Scholar] [CrossRef]
- Thivya, P.; Akalya, S.; Sinija, V.R. A comprehensive review on cellulose-based hydrogel and its potential application in the food industry. Appl. Food Res. 2022, 2, 100161. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Akhila, K.; Ramakanth, D.; Gaikwad, K.K. Guar gum/carboxymethyl cellulose based antioxidant film incorporated with halloysite nanotubes and litchi shell waste extract for active packaging. Int. J. Biol. Macromol. 2022, 201, 1–13. [Google Scholar] [CrossRef]
- Shen, D.; Liu, J.; Gan, L.; Huang, N.; Long, M. Green Synthesis of Fe3O4/Cellulose/Polyvinyl Alcohol Hybride Aerogel and Its Application for Dye Removal. J. Polym. Environ. 2017, 26, 2234–2242. [Google Scholar] [CrossRef]
- Dai, H.; Huang, H. Modified pineapple peel cellulose hydrogels embedded with sepia ink for effective removal of methylene blue. Carbohydr. Polym. 2016, 148, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, J.; Ma, N.; Ma, X.; Gao, M. Bacterial cellulose hydrogel for sensors. Chem. Eng. J. 2023, 461, 142062. [Google Scholar] [CrossRef]
- Salama, A.; Hesemann, P. Recent Trends in Elaboration, Processing, and Derivatization of Cellulosic Materials Using Ionic Liquids. ACS Sustain. Chem. Eng. 2020, 8, 17893–17907. [Google Scholar] [CrossRef]
- Zuppolini, S.; Salama, A.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Cellulose Amphiphilic Materials: Chemistry, Process and Applications. Pharmaceutics 2022, 14, 386. [Google Scholar] [CrossRef]
- Qureshi, D.; Nayak, S.K.; Anis, A.; Ray, S.S.; Kim, D.; Nguyen, T.T.H.; Pal, K. Introduction of biopolymers: Food and biomedical applications. In Biopolymer-Based Formulations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–45. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Selvasekaran, P.; Chidambaram, R. Bioaerogels as food materials: A state-of-the-art on production and application in micronutrient fortification and active packaging of foods. Food Hydrocoll. 2022, 131, 107760. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Zou, P.; Yao, J.; Cui, Y.-N.; Zhao, T.; Che, J.; Yang, M.; Li, Z.; Gao, C. Advances in Cellulose-Based Hydrogels for Biomedical Engineering: A Review Summary. Gels 2022, 8, 364. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Song, X.; Zhao, Y.; Wang, Y.; Rao, L.; Fu, L.; Wang, Z.; Yang, X.; Li, Y.; et al. Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications. Gels 2022, 8, 270. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Jose, G.; Shalumon, K.; Chen, J.-P. Natural Polymers Based Hydrogels for Cell Culture Applications. Curr. Med. Chem. 2020, 27, 2734–2776. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Oliver-Simancas, R.; Castangia, I.; Rodríguez-García, A.M.; Alañón, M.E. Comprehensive review of natural based hydrogels as an upcoming trend for food packing. Food Hydrocoll. 2022, 135, 108124. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Luo, Y.; Wu, T.; Chen, X.; Wang, Y.; Xie, J. Advanced applications of chitosan-based hydrogels: From biosensors to intelligent food packaging system. Trends Food Sci. Technol. 2021, 110, 822–832. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Ajji, A. Moisture absorbers for food packaging applications. Environ. Chem. Lett. 2019, 17, 609–628. [Google Scholar] [CrossRef]
- Lopes, P.M.P.; Moldovan, D.; Fechete, R.; Mare, L.; Barbu-Tudoran, L.; Sechel, N.; Popescu, V. Characterization of a Graphene Oxide-Reinforced Whey Hydrogel as an Eco-Friendly Absorbent for Food Packaging. Gels 2023, 9, 298. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Soleimani, S.; Heydari, A.; Fattahi, M. Swelling prediction of calcium alginate/cellulose nanocrystal hydrogels using response surface methodology and artificial neural network. Ind. Crop. Prod. 2023, 192, 116094. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, J.; Wei, Z.; Zhang, B.; Weng, X. Advances and Progress in Self-Healing Hydrogel and Its Application in Regenerative Medicine. Materials 2023, 16, 1215. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Environ. 2023, 1–25. [Google Scholar] [CrossRef]
- Alam, M.N.; Islam, M.S.; Christopher, L.P. Sustainable Production of Cellulose-Based Hydrogels with Superb Absorbing Potential in Physiological Saline. ACS Omega 2019, 4, 9419–9426. [Google Scholar] [CrossRef]
- Amsden, B.G. Hydrogel Mesh Size and Its Impact on Predictions of Mathematical Models of the Solute Diffusion Coefficient. Macromolecules 2022, 55, 8399–8408. [Google Scholar] [CrossRef]
- Cipriano, B.H.; Banik, S.J.; Sharma, R.; Rumore, D.; Hwang, W.; Briber, R.M.; Raghavan, S.R. Superabsorbent Hydrogels That Are Robust and Highly Stretchable. Macromolecules 2014, 47, 4445–4452. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Zhuang, J.; Xiang, Z.; Jiang, W.; He, S.; Xiao, H. Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges. Polymers 2022, 14, 3739. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Mu, X.; Li, S.; Liu, X.; Lei, Z. Research Progress of Polysaccharide-Based Natural Polymer Hydrogels in Water Purification. Gels 2023, 9, 249. [Google Scholar] [CrossRef]
- Petros, S.; Tesfaye, T.; Ayele, M. A Review on Gelatin Based Hydrogels for Medical Textile Applications. J. Eng. 2020, 2020, 8866582. [Google Scholar] [CrossRef]
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An overview of hydrogels and their role in transdermal drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 574–584. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Vernerey, F.J.; Sridhar, S.L.; Muralidharan, A.; Bryant, S.J. Mechanics of 3D Cell–Hydrogel Interactions: Experiments, Models, and Mechanisms. Chem. Rev. 2021, 121, 11085–11148. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Agnelli, S.; Sartore, L. Designing Viscoelastic Gelatin-PEG Macroporous Hybrid Hydrogel with Anisotropic Morphology and Mechanical Properties for Tissue Engineering Application. Micro 2023, 3, 29. [Google Scholar] [CrossRef]
- Kopač, T.; Ručigaj, A.; Krajnc, M. Effect of polymer-polymer interactions on the flow behavior of some polysaccharide-based hydrogel blends. Carbohydr. Polym. 2022, 287, 119352. [Google Scholar] [CrossRef] [PubMed]
- Camana, G.; Tavano, M.; Li, M.; Castiglione, F.; Rossi, F.; Cellesi, F. Design of Functional Pluronic-Based Precursors for Tailoring Hydrogel Thermoresponsiveness and Cell-Adhesive Properties. Materials 2023, 16, 2749. [Google Scholar] [CrossRef]
- Borges, F.T.P.; Papavasiliou, G.; Teymour, F. Characterizing the Molecular Architecture of Hydrogels and Crosslinked Polymer Networks beyond Flory–Rehner. II: Experiments. Biomacromolecules 2023, 24, 1585–1603. [Google Scholar] [CrossRef]
- Aswathy, S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Lehmann, M.; Krause, P.; Miruchna, V.; von Klitzing, R. Tailoring PNIPAM hydrogels for large temperature-triggered changes in mechanical properties. Colloid Polym. Sci. 2019, 297, 633–640. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Xia, L.-W.; Xie, R.; Ju, X.-J.; Wang, W.; Chen, Q.; Chu, L.-Y. Nano-structured smart hydrogels with rapid response and high elasticity. Nat. Commun. 2013, 4, 2226. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The swollen polymer network hypothesis: Quantitative models of hydrogel swelling, stiffness, and solute transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Wagner, R.J.; Dai, J.; Su, X.; Vernerey, F.J. A mesoscale model for the micromechanical study of gels. J. Mech. Phys. Solids 2022, 167, 100317. [Google Scholar] [CrossRef]
- Pinelli, F.; Magagnin, L.; Rossi, F. Progress in hydrogels for sensing applications: A review. Mater. Today Chem. 2020, 17, 100317. [Google Scholar] [CrossRef]
- Yang, X.; Dargaville, B.L.; Hutmacher, D.W. Elucidating the Molecular Mechanisms for the Interaction of Water with Polyethylene Glycol-Based Hydrogels: Influence of Ionic Strength and Gel Network Structure. Polymers 2021, 13, 845. [Google Scholar] [CrossRef]
- Ting, M.S.; Travas-Sejdic, J.; Malmström, J. Modulation of hydrogel stiffness by external stimuli: Soft materials for mechanotransduction studies. J. Mater. Chem. B 2021, 9, 7578–7596. [Google Scholar] [CrossRef]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Babić, M.M.; Vukomanović, M.; Stefanič, M.; Nikodinović-Runić, J.; Tomić, S.L. Controlled Curcumin Release from Hydrogel Scaffold Platform Based on 2-Hydroxyethyl Methacrylate/Gelatin/Alginate/Iron(III) Oxide. Macromol. Chem. Phys. 2020, 221, 2000186. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Ghorbani, M. Development of a Novel Antibacterial Hydrogel Scaffold Based on Guar Gum/Poly (methylvinylether-alt-maleic Acid) Containing Cinnamaldehyde-Loaded Chitosan Nanoparticles. J. Polym. Environ. 2021, 30, 431–442. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, S.D.; Armir, N.A.Z.; Zulkifli, A.; Hair, A.H.A.; Salleh, K.M.; Lindsey, K.; Che-Othman, M.H.; Zakaria, S. Hydrogel Application in Urban Farming: Potentials and Limitations—A Review. Polymers 2022, 14, 2590. [Google Scholar] [CrossRef] [PubMed]
- Naranda, J.; Bračič, M.; Vogrin, M.; Maver, U. Recent Advancements in 3D Printing of Polysaccharide Hydrogels in Cartilage Tissue Engineering. Materials 2021, 14, 3977. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Ghrayeb, M.; Chai, L. Demonstrating Principle Aspects of Peptide- and Protein- Based Hydrogels Using Metallogels Examples. Isr. J. Chem. 2022, 62, e202200011. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]
- Shahi, S.; Roghani-Mamaqani, H.; Talebi, S.; Mardani, H. Stimuli-responsive destructible polymeric hydrogels based on irreversible covalent bond dissociation. Polym. Chem. 2022, 13, 161–192. [Google Scholar] [CrossRef]
- Jose, J.; Athira, V.; Michel, H.; Hafeela, A.; Bhat, S.G.; Thomas, S.; Maria, L.P. Hydrogels: An overview of the history, classification, principles, applications, and kinetics. Sustain. Hydrogels 2023, 1–22. [Google Scholar] [CrossRef]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2020, 121, 10908–10949. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Mota, L.O.; Gimenez, I.F. Cellulose-Based Materials Crosslinked with Epichlorohydrin: A Mini Review. Rev. Virtual Quím 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef]
- Chaudhary, V.; Bangar, S.P.; Thakur, N.; Trif, M. Recent Advancements in Smart Biogenic Packaging: Reshaping the Future of the Food Packaging Industry. Polymers 2022, 14, 829. [Google Scholar] [CrossRef]
- Singh, A.K.; Kim, J.Y.; Lee, Y.S. Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients. Molecules 2022, 27, 7513. [Google Scholar] [CrossRef]
- Tabassum, Z.; Mohan, A.; Mamidi, N.; Khosla, A.; Kumar, A.; Solanki, P.R.; Malik, T.; Girdhar, M. Recent trends in nanocomposite packaging films utilising waste generated biopolymers: Industrial symbiosis and its implication in sustainability. IET Nanobiotechnol. 2023, 17, 127–153. [Google Scholar] [CrossRef]
- Blažic, R.; Marušić, K.; Vidović, E. Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution. Gels 2023, 9, 94. [Google Scholar] [CrossRef]
- Duceac, I.A.; Stanciu, M.-C.; Nechifor, M.; Tanasă, F.; Teacă, C.-A. Insights on Some Polysaccharide Gel Type Materials and Their Structural Peculiarities. Gels 2022, 8, 771. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Santovito, E.; Cometa, S.; Bevilacqua, A.; Baruzzi, F. Biopolymer hybrid materials: Development, characterization, and food packaging applications. Food Packag. Shelf Life 2021, 28, 100676. [Google Scholar] [CrossRef]
- Gupta, A.; Keddie, D.; Kannappan, V.; Gibson, H.; Khalil, I.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, M.; Shen, J.; He, Z.; Fatehi, P.; Ni, Y. Applications of Cellulose-based Materials in Sustained Drug Delivery Systems. Curr. Med. Chem. 2019, 26, 2485–2501. [Google Scholar] [CrossRef] [PubMed]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef]
- Ghorbani, S.; Eyni, H.; Bazaz, S.R.; Nazari, H.; Asl, L.S.; Zaferani, H.; Kiani, V.; Mehrizi, A.A.; Soleimani, M. Hydrogels Based on Cellulose and its Derivatives: Applications, Synthesis, and Characteristics. Polym. Sci. Ser. A 2018, 60, 707–722. [Google Scholar] [CrossRef]
- Tavakoli, J.; Wang, J.; Chuah, C.; Tang, Y. Natural-based Hydrogels: A Journey from Simple to Smart Networks for Medical Examination. Curr. Med. Chem. 2020, 27, 2704–2733. [Google Scholar] [CrossRef]
- Shi, Z.; Ullah, M.W.; Liang, X.; Yang, G. Recent Developments in Synthesis, Properties, and Biomedical Applications of Cellulose-Based Hydrogels. Nanocellulose Synth. Struct. Prop. Appl. 2021, 121–153. [Google Scholar] [CrossRef]
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Pharm. 2019, 13, 100162. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, S.; Xiong, M.; Lin, F.; Tang, L.; Huang, B.; Chen, Y. One-pot construction of cellulose-gelatin supramolecular hydrogels with high strength and pH-responsive properties. Carbohydr. Polym. 2018, 196, 225–232. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Liu, Z.; Huang, H. Pineapple peel carboxymethyl cellulose/polyvinyl alcohol/mesoporous silica SBA-15 hydrogel composites for papain immobilization. Carbohydr. Polym. 2017, 169, 504–514. [Google Scholar] [CrossRef]
- Masruchin, N.; Park, B.-D.; Causin, V. Influence of sonication treatment on supramolecular cellulose microfibril-based hydrogels induced by ionic interaction. J. Ind. Eng. Chem. 2015, 29, 265–272. [Google Scholar] [CrossRef]
- Wang, G.; Lu, T.; Zhang, X.; Feng, M.; Wang, C.; Yao, W.; Zhou, S.; Zhu, Z.; Ding, W.; He, M. Structure and properties of cellulose/HAP nanocomposite hydrogels. Int. J. Biol. Macromol. 2021, 186, 377–384. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Microencapsulation of betanin by complex coacervation of carboxymethylcellulose and amaranth protein isolate for application in edible gelatin films. Food Hydrocoll. 2022, 133, 107956. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, F.; Zhu, L.; Jiang, J. An in-situ fabrication of bamboo bacterial cellulose/sodium alginate nanocomposite hydrogels as carrier materials for controlled protein drug delivery. Int. J. Biol. Macromol. 2021, 170, 459–468. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Yan, X. Self-assembled supermolecular hydrogel based on hydroxyethyl cellulose: Formation, in vitro release and bacteriostasis application. Carbohydr. Polym. 2017, 172, 49–59. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; He, C.; Mo, X.; Jiang, Y.; Wang, H. Fabrication of antimicrobial films based on hydroxyethylcellulose and ZnO for food packaging application. Food Packag. Shelf Life 2020, 23, 100462. [Google Scholar] [CrossRef]
- Almeida, A.P.; Saraiva, J.N.; Cavaco, G.; Portela, R.P.; Leal, C.R.; Sobral, R.G.; Almeida, P.L. Crosslinked bacterial cellulose hydrogels for biomedical applications. Eur. Polym. J. 2022, 177, 111438. [Google Scholar] [CrossRef]
- Sommer, A.; Dederko-Kantowicz, P.; Staroszczyk, H.; Sommer, S.; Michalec, M. Enzymatic and Chemical Cross-Linking of Bacterial Cellulose/Fish Collagen Composites—A Comparative Study. Int. J. Mol. Sci. 2021, 22, 3346. [Google Scholar] [CrossRef]
- Omer, A.M.; Tamer, T.M.; Hassan, M.E.; Khalifa, R.E.; El-Monaem, E.M.A.; Eltaweil, A.S.; Eldin, M.S.M. Fabrication of Grafted Carboxymethyl Cellulose Superabsorbent Hydrogel for Water Retention and Sustained Release of Ethephon in Sandy Soil. Arab. J. Sci. Eng. 2022, 48, 561–572. [Google Scholar] [CrossRef]
- Tong, R.; Chen, G.; Pan, D.; Qi, H.; Li, R.; Tian, J.; Lu, F.; He, M. Highly Stretchable and Compressible Cellulose Ionic Hydrogels for Flexible Strain Sensors. Biomacromolecules 2019, 20, 2096–2104. [Google Scholar] [CrossRef]
- Pekel, N.; Yoshii, F.; Kume, T.; Güven, O. Radiation crosslinking of biodegradable hydroxypropylmethylcellulose. Carbohydr. Polym. 2004, 55, 139–147. [Google Scholar] [CrossRef]
- Liu, P.; Zhai, M.; Li, J.; Peng, J.; Wu, J. Radiation preparation and swelling behavior of sodium carboxymethyl cellulose hydrogels. Radiat. Phys. Chem. 2002, 63, 525–528. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent Advances in Edible Polymer Based Hydrogels as a Sustainable Alternative to Conventional Polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Tarfdar, A.; Sindhu, R.; Binod, P.; Sirohi, R.; Awasthi, M.K.; Pandey, A. Biorefinery aspects for cost-effective production of nanocellulose and high value-added biocomposites. Fuel 2022, 311, 122575. [Google Scholar] [CrossRef]
- Bayer, I.S. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Sabaghi, M.; Tavasoli, S.; Hoseyni, S.Z.; Mozafari, M.; Degraeve, P.; Katouzian, I. A critical review on approaches to regulate the release rate of bioactive compounds from biopolymeric matrices. Food Chem. 2022, 382, 132411. [Google Scholar] [CrossRef]
- Bolívar-Monsalve, E.J.; Alvarez, M.M.; Hosseini, S.; Espinosa-Hernandez, M.A.; Ceballos-González, C.F.; Sanchez-Dominguez, M.; Shin, S.R.; Cecen, B.; Hassan, S.; Di Maio, E.; et al. Engineering bioactive synthetic polymers for biomedical applications: A review with emphasis on tissue engineering and controlled release. Mater. Adv. 2021, 2, 4447–4478. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Kishida, A.; Ikada, Y. Hydrogels for biomedical and pharmaceutical applications. In Polymeric Biomaterials, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2001; pp. 147–160. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Allahyari, Z.; Jaymand, M. Natural polymers-based light-induced hydrogels: Promising biomaterials for biomedical applications. Coord. Chem. Rev. 2020, 420, 213432. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Regubalan, B.; Pandit, P.; Maiti, S.; Nadathur, G.T.; Mallick, A. Potential Bio-Based Edible Films, Foams, and Hydrogels for Food Packaging. Bio-Based Mater. Food Packag. Green Sustain. Adv. Packag. Mater. 2018, 31, 105–123. [Google Scholar] [CrossRef]
- Kaur, P.; Bohidar, H.B.; Nisbet, D.R.; Pfeffer, F.M.; Rifai, A.; Williams, R.; Agrawal, R. Waste to high-value products: The performance and potential of carboxymethylcellulose hydrogels via the circular economy. Cellulose 2023, 30, 2713–2730. [Google Scholar] [CrossRef]
- Gregorova, A.; Saha, N.; Kitano, T.; Saha, P. Hydrothermal effect and mechanical stress properties of carboxymethylcellulose based hydrogel food packaging. Carbohydr. Polym. 2015, 117, 559–568. [Google Scholar] [CrossRef]
- Samyn, P. Wetting and hydrophobic modification of cellulose surfaces for paper applications. J. Mater. Sci. 2013, 48, 6455–6498. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Hamoud, Y.A.; Xu, X.; Liu, H.; Wang, S.; Sheteiwy, M.; Dong, F.; Guo, L.; Qian, Y.; Li, P.; et al. Thermo-/pH-responsive preservative delivery based on TEMPO cellulose nanofiber/cationic copolymer hydrogel film in fruit packaging. Int. J. Biol. Macromol. 2021, 183, 1911–1924. [Google Scholar] [CrossRef]
- Xie, Y.; Pan, Y.; Cai, P. Cellulose-based antimicrobial films incroporated with ZnO nanopillars on surface as biodegradable and antimicrobial packaging. Food Chem. 2022, 368, 130784. [Google Scholar] [CrossRef]
- Dai, L.; Xi, X.; Li, X.; Li, W.; Du, Y.; Lv, Y.; Wang, W.; Ni, Y. Self-assembled all-polysaccharide hydrogel film for versatile paper-based food packaging. Carbohydr. Polym. 2021, 271, 118425. [Google Scholar] [CrossRef] [PubMed]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis and characterization of superabsorbent hydrogels based on hydroxyethylcellulose and acrylic acid. Carbohydr. Polym. 2017, 166, 300–308. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Alhajaim, W.; Fatima, A.; Yasir, S.; Kamal, T.; Abbas, Y.; Khan, S.; Khan, A.H.; Manan, S.; Ullah, M.W.; et al. Development of low-cost bacterial cellulose-pomegranate peel extract-based antibacterial composite for potential biomedical applications. Int. J. Biol. Macromol. 2023, 231, 123269. [Google Scholar] [CrossRef] [PubMed]
- Tabaght, F.E.; Azzaoui, K.; El Idrissi, A.; Jodeh, S.; Khalaf, B.; Rhazi, L.; Bellaouchi, R.; Asehraou, A.; Hammouti, B.; Sabbahi, R. Synthesis, characterization, and biodegradation studies of new cellulose-based polymers. Sci. Rep. 2023, 13, 1673. [Google Scholar] [CrossRef]
- Dharmalingam, K.; Anandalakshmi, R. Functionalization of cellulose-based nanocomposite hydrogel films with zinc oxide complex and grapefruit seed extract for potential applications in treating chronic wounds. Polymer 2020, 202, 122620. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; Swedish Institute for Food and Biotechnology: Goteborg, Sweden, 2011. [Google Scholar]
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Shanmugam, D.K.; Dasan, A.; Mosas, K.K.A. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef]
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization and applications: A review. Mater. Adv. 2021, 2, 1872–1895. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Sáha, P. Bacterial cellulose and guar gum based modified PVP-CMC hydrogel films: Characterized for packaging fresh berries. Food Packag. Shelf Life 2019, 22, 100402. [Google Scholar] [CrossRef]
- Pirsa, S. Nanocomposite base on carboxymethylcellulose hydrogel: Simultaneous absorbent of ethylene and humidity to increase the shelf life of banana fruit. Int. J. Biol. Macromol. 2021, 193, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.; Burdiles, P.A.; Quero, F.; Palma, P.; Olate-Moya, F.A.; Palza, H. 3D Printing of Antimicrobial Alginate/Bacterial-Cellulose Composite Hydrogels by Incorporating Copper Nanostructures. ACS Biomater. Sci. Eng. 2019, 5, 6290–6299. [Google Scholar] [CrossRef]
- Lu, P.; Zhao, H.; Zheng, L.; Duan, Y.; Wu, M.; Yu, X.; Yang, Y. Nanocellulose/Nisin Hydrogel Microparticles as Sustained Antimicrobial Coatings for Paper Packaging. ACS Appl. Polym. Mater. 2022, 4, 2664–2673. [Google Scholar] [CrossRef]
- Sutthasupa, S.; Padungkit, C.; Suriyong, S. Colorimetric ammonia (NH3) sensor based on an alginate-methylcellulose blend hydrogel and the potential opportunity for the development of a minced pork spoilage indicator. Food Chem. 2021, 362, 130151. [Google Scholar] [CrossRef]
- Lu, P.; Liu, R.; Liu, X.; Wu, M. Preparation of Self-supporting Bagasse Cellulose Nanofibrils Hydrogels Induced by Zinc Ions. Nanomaterials 2018, 8, 800. [Google Scholar] [CrossRef]
- Mahmud, J.; Sarmast, E.; Shankar, S.; Lacroix, M. Advantages of nanotechnology developments in active food packaging. Food Res. Int. 2022, 154, 111023. [Google Scholar] [CrossRef]
- Anvar, A.A.; Ahari, H.; Ataee, M. Antimicrobial Properties of Food Nanopackaging: A New Focus on Foodborne Pathogens. Front. Microbiol. 2021, 12, 690706. [Google Scholar] [CrossRef]
- Pirsa, S.; Chavoshizadeh, S. Design of an optical sensor for ethylene based on nanofiber bacterial cellulose film and its application for determination of banana storage time. Polym. Adv. Technol. 2018, 29, 1385–1393. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Li, X.; Lou, J.; Ding, Q.; Liu, Z.; Jiang, Y.; Han, W. Bacterial cellulose-based hydrogel thermocells for low-grade heat harvesting. Chem. Eng. J. 2022, 433, 134550. [Google Scholar] [CrossRef]
- Luo, X.; Zaitoon, A.; Lim, L.T. A review on colorimetric indicators for monitoring product freshness in intelligent food packaging: Indicator dyes, preparation methods, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2489–2519. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Health Mater. 2021, 10, e2100062. [Google Scholar] [CrossRef] [PubMed]




| Synthesis Routes | Crosslinking Mechanism | Types of Cellulose | Characteristics | References |
|---|---|---|---|---|
| Physical crosslinking | Freeze thawing | Native cellulose | One-pot supramolecular bio-based hydrogels with high strength and pH sensitivity | [105] |
| Carboxymethyl cellulose (CMC) | An eco-friendly method of repeated freeze–thaw cycles to develop hydrogel composites based on pineapple peel CMC, polyvinyl alcohol, and mesoporous silica SBA-15 | [106] | ||
| Ionic interaction | Cellulose microfibrils | Synthesis of cellulose microfibrils hydrogels using the TEMPO-oxidation system with increased storage modulus, compression strength, and surface area | [107] | |
| Nanocellulose | Development of sugarcane bagasse nanocellulose-based hydrogel as a colorimetric freshness indicator for detecting chicken breast deterioration | [38] | ||
| Hydrogen-bonding interaction | Native cellulose | Facile and low-cost cellulose-based nanocomposite hydrogels with improved mechanical characteristics and adsorption to heavy metal ions utilizing hydroxyapatite (HAP) nanoparticles | [108] | |
| Complex coacervation | Carboxymethyl cellulose (CMC) | Amaranth protein isolate/CMC complex coacervates develop betanin-containing microcapsules for the creation of edible gelatin films with low light transmission and high antioxidant activity | [109] | |
| Hydrophobic interaction | Bacterial cellulose (BC) | Synthesis of a sodium alginate-bacterial cellulose-based nanocomposite hydrogel with multi-layered porous surfaces capable of swelling, releasing, and being biocompatible for substrate use | [110] | |
| Chemical crosslinking | Grafting | Hydroxyethyl cellulose (HEC) | Synthesis of self-assembled supermolecular hydrogels based on HEC with potential applications as bacteriostasis materials | [111] |
| Crosslinking agents | Carboxymethyl cellulose (CMC) | Crosslinked CMC/gelatin hydrogel films loaded with ZnO nanoparticles using glutaraldehyde as a crosslinking agent with antibacterial and antioxidant characteristics for sustainable food packaging applications | [12] | |
| Hydroxyethyl cellulose (HEC) | Development of citric acid cross-linked antimicrobial hydrogel films based on HEC and ZnO for food packaging applications | [112] | ||
| Bacterial cellulose (BC) | Crosslinked bacterial cellulose hydrogels with improved mechanical properties and increased water retention capacity employing citric acid and epichlorohydrin as crosslinking agents | [113] | ||
| Enzyme crosslinking | Bacterial cellulose (BC) | Transglutaminase-enzymatic crosslinking of BC/fish collagen composites with increased tensile strength and water vapor permeability | [114] | |
| Radical polymerization | Carboxymethyl cellulose (CMC) | Fabrication of a superabsorbent hydrogel for water retention and sustained release in advanced agricultural applications | [115] | |
| Native cellulose | The synthesis of remarkably stretchable and compressible cellulose ionic hydrogels for flexible strain sensors | [116] | ||
| Radiation crosslinking | Hydroxypropyl Methylcellulose (HPMC) | Developed biodegradable HPMC hydrogels with increased strength and swelling qualities using high-energy radiation from electron accelerators. | [117] | |
| Carboxymethyl cellulose (CMC) | An effective method for synthesizing CMC hydrogels with tailored swelling behavior by varying the radiation dose and the degree of carboxymethylation for targeted applications | [118] |
| Property | Main Characteristics | Analytical Techniques | Significance | References |
|---|---|---|---|---|
| Swelling index | Assessment of CBH performance in fluid absorption and swelling behavior | Gravimetric analysis; swelling ratio | Effectiveness of the packaging in preserving the food product by absorbing excess amount of liquid, typically water, and creating a protective barrier around the food product. | [134,136] |
| Wettability | Examining the degree of interaction between CBHs surfaces and fluids | Contact angle measurement; surface energy measurement; (AFM) | Consideration of wettability of CBHs surface for designing food packaging materials with emphasis on barrier properties and prevention of food product loss or contamination | [137] |
| Mechanical strength | Investigation of CBHs endurance and performance under specific environmental constraints | Tensile strength; elongation at break; compression strength; rheological characteristics | Preventing physical damage during handling, transportation, and storage by effectively protecting the food product while maintaining the structural integrity and functionality of packaging material under diverse loading conditions | [17,138] |
| Thermal stability | Thermal analysis under specific temperature conditions | Thermogravimetric analysis (TGA); differential scanning calorimetry (DSC) | Essential for designing food packaging materials to withstand high temperatures during processing, storage, and transportation without compromising the quality and safety of the food product | [12,17] |
| Physical/morphological characterization | Visualizing the structural features of CBH networks | Scanning electron microscopy (SEM); AFM; field emission scanning electron microscopy (FESEM) | Designing suitable food packaging materials with the optimal porosity, barrier characteristics, and mechanical strength to maintain food quality and safety by visualizing the structural aspects of CBH networks | [134,139,140] |
| Chemical characterization | Identifying the chemical structures, molecular arrangements, and functional groups of the developed CBHs | Fourier transform infrared (FTIR) spectroscopy; X-ray diffraction (XRD); (NMR) spectroscopy; Raman spectroscopy | Optimizing the performance of CBHs using chemical composition, degree of crystallinity, and molecular orientation of the hydrogels | [134,141,142] |
| Packaging Type | Type of Cellulose | Combinations | Characteristics | Applications | References |
|---|---|---|---|---|---|
| Hydrogel film | Carboxymethyl cellulose | Polyvinyl alcohol(PVA)/Poly(ethylene imine) (PEI)/Tannic acid (TA) | Hydrogel film with exceptional mechanical strength, self-healing, UV-blocking, strong adhesive strength, antioxidation advantages, and barrier characteristics | Mangoes, strawberries, and cherries | [17] |
| Hydrogel film | Bacterial cellulose | Polyvinyl pyrrolidone (PVP)/CMC/Guar gum | The remarkable elastic and load-bearing capacity of developed hydrogel films, as well as excellent barrier property and shelf life-enhancing properties of berries up to 15 days | Blueberries | [150] |
| Hydrogel film | Carboxymethyl cellulose | Gelatin (GEL)/ZnO-NPs | Sustainable hydrogel films with better mechanical properties and good thermal stability with antibacterial activity and antioxidant properties against two food pathogens, Staphylococcus aureus and Escherichia coli | - | [12] |
| Hydrogel film | Carboxymethyl cellulose | Cellulose nanofiber/Potassium permanganate | Implementing active hydrogels to enhance the shelf life of bananas by performing as a humidity/ethylene absorbent in the developed food packaging film | Banana | [151] |
| Hydrogel composite | Bacterial cellulose | Alginate/Cu-NPs | Innovative antimicrobial 3D-printed hydrogel composite that exhibits excellent printability and antibacterial behavior against Escherichia coli and Staphylococcus aureus | - | [152] |
| Hydrogel coating | TEMPO-oxidized bagasse cellulose nanofibrils (CNF) | Nisin | Significant reduction in the development of Listeria monocytogenes on the external layer of cheese using the developed antimicrobial hydrogel microparticle coating as a paper packaging material | Cheese | [153] |
| Hydrogel Indicator | Methyl cellulose | Alginate/Bromothymol blue | Hydrogel-based spoilage indicator for minced pork storage: color change upon detection of total volatile basic nitrogen (TVB-N) build-up at 4 °C, demonstrating potential for intelligent packaging applications | Minced pork | [154] |
| Hydrogel Indicator | TEMPO-oxidized bagasse cellulose nanofibrils (CNF) | Bromothymol blue/Methyl red | Self-supporting CO2-sensitive cellulose hydrogel as a colorimetric indicator for food deterioration in intelligent food packaging application | Fresh-cut fruits | [155] |
| Hydrogel Indicator | Bagasse nanocellulose | Bromothymol blue/Methyl red | Developed hydrogel as a pH-responsive dye carrier used as a colorimetric freshness indicator to track the degradation of chicken breasts as the amount of CO2 increased in the headspace | Chicken breasts | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.K.; Itkor, P.; Lee, Y.S. State-of-the-Art Insights and Potential Applications of Cellulose-Based Hydrogels in Food Packaging: Advances towards Sustainable Trends. Gels 2023, 9, 433. https://doi.org/10.3390/gels9060433
Singh AK, Itkor P, Lee YS. State-of-the-Art Insights and Potential Applications of Cellulose-Based Hydrogels in Food Packaging: Advances towards Sustainable Trends. Gels. 2023; 9(6):433. https://doi.org/10.3390/gels9060433
Chicago/Turabian StyleSingh, Ajit Kumar, Pontree Itkor, and Youn Suk Lee. 2023. "State-of-the-Art Insights and Potential Applications of Cellulose-Based Hydrogels in Food Packaging: Advances towards Sustainable Trends" Gels 9, no. 6: 433. https://doi.org/10.3390/gels9060433