Clotrimazole-Loaded Borneol-Based In Situ Forming Gel as Oral Sprays for Oropharyngeal Candidiasis Therapy
Abstract
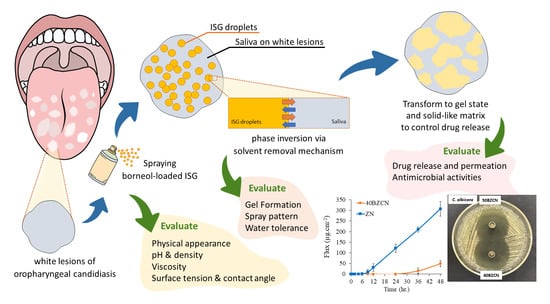
:1. Introduction
2. Results and Discussion
2.1. Physical Appearance
2.2. pH and Density
2.3. Surface Tension
2.4. Viscosity and Rheological Behavior
2.5. Contact Angle
2.6. Microscopic Changes of In Vitro Gel Formation
2.7. Macroscopic Changes of In Vitro Gel Formation
2.8. Spray Pattern
2.9. Water Tolerance
2.10. Drug Release and Permeation
2.11. Antimicrobial Activities
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the ISGs
4.3. Evaluations
4.3.1. pH, Density, and Viscosities
4.3.2. Surface Tension and Contact Angle
4.3.3. Spray Pattern
4.3.4. Water Tolerance Measurement
4.3.5. Gel-Formation Study
4.3.6. In Vitro Drug Release, Permeation, and Drug Retention Studies
4.3.7. Antimicrobial Activity
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayuningtyas, N.F.; Mahdani, F.Y.; Pasaribu, T.A.S.; Chalim, M.; Ayna, V.K.P.; Santosh, A.B.R.; Santacroce, L.; Surboyo, M.D.C. Role of Candida albicans in oral carcinogenesis. Pathophysiology 2022, 29, 51. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Ninane, J.; Multicentre Study Group. A multicentre study of fluconazole versus oral polyenes in the prevention of fungal infection in children with hematological or oncological malignancies. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, C.L. Candidiasis (oropharyngeal). BMJ Clin. Evid. 2013, 2013, 1304. [Google Scholar]
- Ellepola, A.N.B.; Samaranayake, L.P. Antimycotic agents in oral candidosis: An overview: 1. Clinical variants. Dental Update 2000, 27, 111–116. [Google Scholar] [CrossRef]
- Oropharyngeal Fungal Infections. Available online: https://bnf.nice.org.uk/treatment-summary/oropharyngeal-fungal-infections.html (accessed on 23 October 2020).
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Clotrimazole. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2812 (accessed on 4 March 2023).
- Hoogerheide, J.G.; Wyka, B.E. Clotrimazole. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Cambridge, MA, USA, 1982; pp. 225–255. [Google Scholar]
- Crowley, P.D.; Gallagher, H.C. Clotrimazole as a pharmaceutical: Past, present and future. J. Appl. Microbiol. 2014, 117, 611–617. [Google Scholar] [CrossRef]
- Paradkar, M.; Thakkar, V.; Soni, T.; Gandhi, T.; Gohel, M. Formulation and evaluation of clotrimazole transdermal spray. Drug. Dev. Ind. Pharm. 2015, 41, 1718–1725. [Google Scholar] [CrossRef]
- Sawyer, P.R.; Brogden, R.N.; Pinder, K.M.; Speight, T.M.; Avery, G.S. Clotrimazole: A review of its antifungal activity and therapeutic efficacy. Drugs 1975, 9, 424–447. [Google Scholar] [CrossRef]
- Marek, C.L.; Timmons, S.R. 9-antimicrobials in pediatric dentistry. In Pediatric Dentistry, 6th ed.; Nowak, A.J., Christensen, J., Mabry, T., Townsend, J., Wells, M., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 128–141.e1. [Google Scholar]
- Moon, S.E.; Kim, H.Y.; Cha, J.D. Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch. Oral. Biol. 2011, 56, 907–916. [Google Scholar] [CrossRef]
- Gijzen, M.; Lewinsohn, E.; Savage, T.J.; Croteau, R.B. Conifer monoterpenes: Biochemistry and bark beetle chemical ecology. In Bioactive Volatile Compounds from Plants; Teranishi, R., Buttery, R.G., Sugisawa, H., Eds.; American Chemistry Society: Washington, DC, USA, 1991. [Google Scholar]
- Gislene, G.F.; Paulo, C.; Giuliana, L. Antibacterial activity of plant extracts and phytochemicals on antibiotic resistant bacteria. Braz. J. Microbiol. 2000, 31, 314–325. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1995, 86, 985–990. [Google Scholar] [CrossRef]
- Mahadlek, J.; Charoenteeraboon, J.; Phaechamud, T. Zinc oxide gels for periodontitis treatment. J. Metal. Mater. Mineral. 2010, 20, 159–163. [Google Scholar]
- Denyer, S.P.; Hugo, W.B. Biocide-induced damage to the bacterial cytoplasmic membrane. In Mechanism of Action of Chemical Biocides; Denyer, S.P., Hugo, W.B., Eds.; Blackwell Scientific Publications: Oxford, UK, 1991; pp. 171–188. [Google Scholar]
- Sheshala, R.; Hong, G.C.; Yee, W.P.; Meka, V.S.; Thakur, R.R.S. In situ forming phase-inversion implants for sustained ocular delivery of triamcinolone acetonide. Drug Deliv. Transl. Res. 2019, 9, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. In-situ forming PLGA implants for intraocular dexamethasone delivery. Int. J. Pharm. 2018, 548, 337–348. [Google Scholar] [CrossRef]
- Phaechamud, T.; Setthajindalert, O. Antimicrobial in-situ forming gels based on bleached shellac and different solvents. J. Drug Deliv. Sci. Technol. 2018, 46, 285–293. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-based antisolvent-induced in situ forming matrix for crevicular pocket delivery of vancomycin hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on borneol. Food Chem. Toxicol. 2008, 46 (Suppl. 11), S77–S80. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Kromidas, L.; La Cava, S.; et al. RIFM fragrance ingredient safety assessment, borneol, CAS registry number 507-70-0. Food Chem. Toxicol. 2015, 82, S81–S88. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Almeida, J.R.; Souza, G.R.; Silva, J.C.; Saraiva, S.R.; Júnior, R.G.; Quintans, J.D.; Barreto, R.D.; Bonjardim, L.R.; Cavalcanti, S.C.; Junior, L.J. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013, 2013, 808460. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.P.; Gao, X.C.; Zhang, L.Q.; Wei, S.Q.; Bi, S.; Yang, Z.C.; Cui, H. In vitro evaluation of enhancing effect of borneol on transcorneal permeation of compounds with different hydrophilicities and molecular sizes. Eur. J. Pharmacol. 2013, 705, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Senarat, S.; Lwin, W.W.; Mahadlek, J.; Phaechamud, T. Doxycycline hyclate-loaded in situ forming gels composed from bleached shellac, Ethocel, and Eudragit RS for periodontal pocket delivery. Saudi Pharm. J. 2021, 29, 252–263. [Google Scholar] [CrossRef]
- Criado, C.; Muñoz-González, C.; Mora, M.; Fernández-Ruíz, V.; Chaya, C.; Pozo-Bayón, M.A. Understanding if differences in salivary flow rate and total protein content triggered by biological factors (sex and age) affect aroma perception and the hedonic and emotional response of wine consumers. Foods 2022, 11, 3104. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press L.L.C.: Boca Raton, FL, USA, 2014; pp. 3–58. [Google Scholar]
- Qazi, M.J.; Schlegel, S.J.; Backus, E.G.H.; Bonn, M.; Bonn, D.; Shahidzadeh, N. Dynamic surface tension of surfactants in the presence of high salt concentrations. Langmuir 2020, 36, 7956–7964. [Google Scholar] [CrossRef] [PubMed]
- Golmaghani-Ebrahimi, E.; Bagheri, A.; Fazli, M. The influence of temperature on surface concentration and interaction energy between components in binary liquid systems. J. Chem. Thermodyn. 2020, 146, 10615. [Google Scholar] [CrossRef]
- Ershad, A.L.; Rajabi-Siahboomi, A.; Missaghi, S.; Kirby, D.; Mohammed, A.R. Multi-analytical framework to assess the in vitro swallowability of solid oral dosage forms targeting patient. Pharmaceutics 2021, 13, 411. [Google Scholar] [CrossRef]
- Phaechamud, T.; Jantadee, T.; Mahadlek, J.; Charoensuksai, P.; Pichayakorn, W. Characterization of antimicrobial agent loaded eudragit RS solvent exchange-induced in situ forming gels for periodontitis treatment. AAPS PharmSciTech 2017, 18, 494–508. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J.; Chuenbarn, T. In situ forming gel comprising bleached shellac loaded with antimicrobial drugs for periodontitis treatment. Mater. Des. 2016, 89, 294–303. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J. Solvent exchange-induced in situ forming gel comprising ethyl cellulose-antimicrobial drugs. Int. J. Pharm. 2015, 494, 381–392. [Google Scholar] [CrossRef]
- Phaechamud, T.; Thurein, S.M.; Chantadee, T. Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel. Asian J. Pharm. Sci. 2018, 13, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Basma, N.S.; Headen, T.F.; Shaffer, M.S.P.; Skipper, N.T.; Howard, C.A. Local structure and polar order in liquid N-methyl-2-pyrrolidone (NMP). J. Phys. Chem. B 2018, 122, 8963–8971. [Google Scholar] [CrossRef] [PubMed]
- Khaing, E.M.; Mahadlek, J.; Okonogi, S.; Phaechamud, T. Lime peel oil-incorporated rosin-based antimicrobial in situ forming gel. Gels 2022, 8, 169. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Natural-resin in-situ-forming gels: Physicochemical characteristics and bioactivities. Pharm. Sci. Asia 2021, 48, 461–470. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Sirirak, J.; Tuntarawongsa, S.; Okonogi, S.; Phaechamud, T. Design and comparative evaluation of vancomycin HCl-loaded rosin-based in situ forming gel and microparticles. Gels 2022, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Puyathorn, N.; Senarat, S.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical and bioactivity characteristics of doxycycline hyclate-loaded solvent removal-induced ibuprofen-based in situ forming gel. Gels 2023, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Saturated fatty acid-based in situ forming matrices for localized antimicrobial delivery. Pharmaceutics 2020, 12, 808. [Google Scholar] [CrossRef]
- Chantadee, T.; Sawangsri, P.; Santimaleeworagun, W.; Phaechamud, T. Vancomycin hydrochloride-loaded stearic acid/lauric acid in situ forming matrix for antimicrobial inhibition in patients with joint infection after total knee arthroplasty. Mat. Sci. Eng. C 2020, 115, 110761. [Google Scholar] [CrossRef]
- Chantadee, T.; Sirirak, J.; Hoshino, T.; Phaechamud, T. Augmentative molecular aspect for phase inversion of vancomycin hydrochloride-loaded fatty acid in situ forming matrices. Mater. Des. 2021, 199, 109429. [Google Scholar] [CrossRef]
- Zhang, Q.; Fassihi, M.A.; Fassihi, R. Delivery considerations of highly viscous polymeric fluids mimicking concentrated biopharmaceuticals: Assessment of injectability via measurement of total work done “WT”. AAPS Pharm. Sci. Tech. 2018, 19, 1520–1528. [Google Scholar] [CrossRef]
- Senarat, S.; Cheunban, T.; Phaechamud, T.; Chantadee, T. Interfacial properties of rosin solution for the development of natural resin based in situ forming systems. Mater. Today Proc. 2023, 75, 58–66. [Google Scholar] [CrossRef]
- Dwivedi, R.K.; Jain, V.; Muralidhar, K. Dynamic contact angle model for resolving low-viscosity droplet oscillations during spreading over a surface with varying wettability. Phys. Rev. Fluids 2022, 7, 034002. [Google Scholar] [CrossRef]
- Senarat, S.; Charoenteeraboon, J.; Praphanwittaya, P.; Phaechamud, T. Phase behavior of doxycycline hyclate-incorporated bleached shellac in-situ forming gel/microparticle after solvent movement. Key Eng. Mater. 2020, 859, 21–26. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.M.; Stapley, A.G. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid. Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef]
- Yamamoto, S. Drying of gelled sugar solutions-water diffusion behavior. Chem. Eng. J. 2002, 86, 179–184. [Google Scholar] [CrossRef]
- Yang, L.J.; Yang, X.Q.; Huang, K.M.; Jia, G.Z.; Shang, H. Dielectric properties of binary solvent mixtures of dimethyl sulfoxide with water. Int. J. Mol. Sci. 2009, 10, 1261–1270. [Google Scholar] [CrossRef]
- Iamir, E.M.; Ibrahim, S.Y. Physical Properties of aqueous N-methyl pyrrolidone at different temperatures. Pet. Sci. Technol. 2004, 22, 1571–1579. [Google Scholar] [CrossRef]
- Höfs, S.; Mogavero, S.; Hube, B. Interaction of Candida albicans with host cells: Virulence factors, host defense, escape strategies and the microbiota. J. Microbiol. 2016, 53, 149–169. [Google Scholar] [CrossRef]
- Phan, Q.T.; Belanger, P.H.; Filler, S.G. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect. Immun. 2000, 68, 3485–3490. [Google Scholar] [CrossRef]
- Swidergall, M.; Filler, S.G. Oropharyngeal candidiasis: Fungal invasion and epithelial cell responses. PLoS Pathog. 2017, 13, e1006056. [Google Scholar] [CrossRef]
- Sikandar, M.; Shoaib, M.H.; Yousuf, R.I.; Ahmed, F.R.; Ali, F.R.; Saleem, M.T.; Ahmed, K.; Sarfaraz, S.; Jabeen, S.; Siddiqui, F.; et al. Nanoclay-based composite films for transdermal drug delivery: Development, characterization, and in silico modeling and simulation. Int. J. Nanomed. 2022, 17, 3463–3481. [Google Scholar] [CrossRef] [PubMed]
- Hashem, F.M.; Shaker, D.S.; Ghorab, M.K.; Nasr, M.; Ismail, A. Formulation, characterization, and clinical evaluation of microemulsion containing clotrimazole for topical delivery. AAPS PharmSciTech 2011, 12, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An updated overview of the emerging role of patch and film-based buccal delivery systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef] [PubMed]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved topical drug delivery: Role of permeation enhancers and advanced approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Darío, A.; Tinjacá, F.M.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Effect of N-methyl-pyrrolidone (NMP) on the equilibrium solubility of meloxicam in aqueous media: Correlation, dissolution thermodynamics, and preferential solvation. ACS Omega 2022, 7, 37988–38002. [Google Scholar] [CrossRef]
- Büchter, A.; Meyer, U.; Kruse-Lösler, B.; Joos, U.; Kleinheinz, J. Sustained release of doxycycline for the treatment of peri-implantitis: Randomised controlled trial. Br. J. Oral. Maxillofac. Surg. 2004, 42, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Mahadlek, J.; Charoenteeraboon, J.; Choopun, S. Characterization and antimicrobial activity of N-methyl-2-pyrrolidone-loaded ethylene oxide-propylene oxide block copolymer thermosensitive gel. Indian J. Pharm. Sci. 2012, 74, 498. [Google Scholar] [CrossRef]
- Alanazi, A.K.; Alqasmi, M.H.; Alrouji, M.; Kuriri, F.A.; Almuhanna, Y.; Joseph, B.; Asad, M. Antibacterial activity of syzygium aromaticum (clove) bud oil and its interaction with imipenem in controlling wound infections in rats caused by methicillin-resistant Staphylococcus aureus. Molecules 2022, 27, 8551. [Google Scholar] [CrossRef]
- Biernasiuk, A.; Baj, T.; Malm, A. Clove essential oil and its main constituent, eugenol, as potential natural antifungals against Candida spp. alone or in combination with other antimycotics due to synergistic interactions. Molecules 2023, 28, 215. [Google Scholar] [CrossRef]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58 Pt 11, 1454–1462. [Google Scholar] [CrossRef]
- Rein, S.M.T.; Mahadlek, J.; Charoenteeraboon, J.; Phaechamud, T. Clotrimazole-incorporated fatty acid-based in situ forming film containing pressure sensitive adhesive. Mat. Today Proc. 2022, 65 Pt 40, 2277–2283. [Google Scholar]
- Mahadlek, J.; Rein, S.M.T.; Thammasut, W.; Phaechamud, T. Clotrimazole-loaded fatty acid-based in situ forming film oral spray. Mater. Today Proc. 2022, 52 Pt 5, 2479–2484. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zhan, F. Effects of natural borneol on germ tube formation and preformed biofilm activity in Candida albicans. Nat. Prod. Commun. 2022, 17, 1–5. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Sonawane, S.J.; Sikwal, D.R.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Solid lipid nanoparticles of clotrimazole silver complex: An efficient nano antibacterial against Staphylococcus aureus and MRSA. Colloids Surf. B 2015, 136, 651–658. [Google Scholar] [CrossRef]
- Mendes, S.S.; Marques, J.; Mesterházy, E.; Straetener, J.; Arts, M.; Pissarro, T.; Reginold, J.; Berscheid, A.; Bornikoel, J.; Kluj, R.M.; et al. Synergetic antimicrobial activity and mechanism of clotrimazole-linked CO-releasing molecules. ACS Bio Med Chem Au 2022, 2, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Frej-Mądrzak, M.; Golec, S.; Włodarczyk, K.; Choroszy-Król, I.; Nawrot, U. Susceptibility to clotrimazole of Candida spp. Isolated from the genitourinary system-a single center study. Pathogens 2021, 10, 1142. [Google Scholar] [CrossRef]
- Lagerlof, F.; Dawes, C. The volume of saliva in the mouth before and after swallowing. J. Dent. Res. 1984, 63, 618–621. [Google Scholar] [CrossRef]
- Luo, L.; Li, G.; Luan, D.; Yuan, Q.; Wei, Y.; Wang, X. Antibacterial adhesion of borneol-based polymer via surface chiral stereochemistry. ACS Appl. Mater. Interfaces 2014, 6, 19371–19377. [Google Scholar] [CrossRef]








| Formulation | pH | Density (g/cm3) | Surface Tension (mN/m) | Viscosity (cPs) |
|---|---|---|---|---|
| NMP | 11.07 ± 0.07 | 1.03 ± 0.01 | 37.36 ± 0.74 | 2.40 ± 0.06 |
| CN | 10.68 ± 0.04 | 1.03 ± 0.01 | 37.47 ± 0.79 | 2.54 ± 0.07 |
| ZN | 7.59 ± 0.01 a,b | 1.03 ± 0.00 | 38.34 ± 0.95 | 2.62 ± 0.10 |
| ZCN | 7.43 ± 0.02 a,b | 1.03 ± 0.00 | 37.77 ± 0.86 | 2.44 ± 0.03 |
| 20BZCN | 6.61 ± 0.01 a,b | 1.02 ± 0.01 | 33.32 ± 0.93 c | 3.83 ± 0.02 d |
| 30BZCN | 6.22 ± 0.01 a,b | 1.01 ± 0.01 | 32.39 ± 1.10 c | 4.31 ± 0.01 d |
| 40BZCN | 5.89 ± 0.02 a,b | 1.01 ± 0.00 | 31.42 ± 0.92 c | 5.35 ± 0.03 d |
| 50BZCN | 5.59 ± 0.01 a,b | 1.00 ± 0.01 | 30.19 ± 0.61 c | 7.91 ± 0.11 d |
| 40BN | 5.91 ± 0.03 a,b | 1.01 ± 0.01 | 32.19 ± 0.70 c | 5.02 ± 0.05 d |
| Place of Drug for Analyzing | Clotrimazole Remains (%) | |
|---|---|---|
| 40BZCN | ZN | |
| Borneol matrix over buccal porcine (Donor chamber) | 77.10 ± 5.72 * | N/A |
| Inside buccal porcine (Membrane) | 16.39 ± 5.65 * | 75.51 ± 9.81 * |
| Under buccal porcine (Receptor chamber) | 3.44 ± 1.56 | 21.56 ± 4.73 |
| Microbes | Inhibition Zone Diameter (Mean ± SD) (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| NMP | CN | ZN | ZCN | 20BZCN | 30BZCN | 40BZCN | 50BZCN | |
| S. aureus ATCC 6538 | 10.67 ± 1.53 | 14.17 ± 1.04 | 21.33 ± 1.53 | 20.00 ± 1.00 | 20.33 ± 0.58 | 17.00 ± 1.00 | 16.00 ± 1.00 | 13.83 ± 1.26 |
| E. coli ATCC 25922 | 17.67 ± 1.53 | 19.00 ± 1.00 | 18.00 ± 1.00 | 17.00 ± 1.00 | 14.00 ± 1.00 | 12.33 ± 1.53 | 11.00 ± 1.00 | 8.17 ± 0.29 |
| C. albicans ATCC 17110 | 28.33 ± 1.53 | 30.33 ± 0.58 | 39.33 ± 0.58 | 39.00 ± 1.00 | 43.67 ± 1.15 | 42.67 ± 0.58 a | 42.33 ± 0.58 a | 42.67 ± 0.58 a |
| C. krusei TISTR 5259 | 14.33 ± 1.53 | 14.00 ± 2.00 | 37.00 ± 2.00 b | 32.00 ± 1.00 | 38.67 ± 0.58 | 36.33 ± 1.53 b | 34.67 ± 1.15 | 31.00 ± 1.73 |
| C. lusitaniae TISTR 5156 | 30.00 ± 1.00 | 30.67 ± 0.58 | 38.33 ± 1.15 c | 38.33 ± 0.58 c | 39.33 ± 1.53 | 37.00 ± 1.00 c | 35.33 ± 0.58 | 33.33 ± 0.58 |
| C. tropicalis TISTR 5306 | 27.67 ± 0.58 | 29.33 ± 0.58 | 36.33 ± 0.58 | 37.00 ± 1.00 | 40.67 ± 1.15 | 39.33 ± 0.58 d | 36.33 ± 0.58 | 35.00 ± 1.00 |
| Formulation | Amount (%w/w) | |||
|---|---|---|---|---|
| Clotrimazole | Clove oil | Borneol | NMP | |
| NMP | - | - | - | 100 |
| CN | - | 1 | - | 99 |
| ZN | 1 | - | - | 99 |
| ZCN | 1 | 1 | - | 98 |
| 20BZCN | 1 | 1 | 20 | 78 |
| 30BZCN | 1 | 1 | 30 | 68 |
| 40BZCN | 1 | 1 | 40 | 58 |
| 50BZCN | 1 | 1 | 50 | 48 |
| 40BN | - | - | 40 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lertsuphotvanit, N.; Tuntarawongsa, S.; Jitrangsri, K.; Phaechamud, T. Clotrimazole-Loaded Borneol-Based In Situ Forming Gel as Oral Sprays for Oropharyngeal Candidiasis Therapy. Gels 2023, 9, 412. https://doi.org/10.3390/gels9050412
Lertsuphotvanit N, Tuntarawongsa S, Jitrangsri K, Phaechamud T. Clotrimazole-Loaded Borneol-Based In Situ Forming Gel as Oral Sprays for Oropharyngeal Candidiasis Therapy. Gels. 2023; 9(5):412. https://doi.org/10.3390/gels9050412
Chicago/Turabian StyleLertsuphotvanit, Nutdanai, Sarun Tuntarawongsa, Kritamorn Jitrangsri, and Thawatchai Phaechamud. 2023. "Clotrimazole-Loaded Borneol-Based In Situ Forming Gel as Oral Sprays for Oropharyngeal Candidiasis Therapy" Gels 9, no. 5: 412. https://doi.org/10.3390/gels9050412