Multifunctional Finishing of Cotton Fabric with Curcumin Derivatives Coatings Obtained by Sol–Gel Method
Abstract
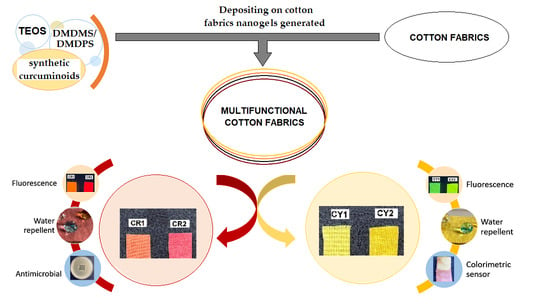
:1. Introduction
2. Results and Discussion
2.1. Structure and Morphology of the Coated Fabrics
2.2. Optical Properties of the Coated Fabrics
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Obtaining and the Method of Depositing Nanosols
4.2.2. Characterization Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, M.; Nayem, K.A.; Azim, A.Y.M.A. Dyeing of Cotton and Silk Fabric with Purified Natural Curcumin Dye. Int. Res. J. Eng. Technol. 2014, 3, 838–844. [Google Scholar]
- Sfameni, S.; Hadhri, M.; Rando, G.; Drommi, D.; Rosace, G.; Trovato, V.; Plutino, M.R. Inorganic Finishing for Textile Fabrics: Recent Advances in Wear-Resistant, UV Protection and Antimicrobial Treatments. Inorganics 2023, 11, 19. [Google Scholar] [CrossRef]
- Radhika, D. Review Study on Antimicrobial Finishes on Textiles—Plant Extracts and Their Application. Int. Res. J. Eng. Technol. 2019, 6, 3581–3588. [Google Scholar]
- Muhammad, N. Potential Application Techniques for Antimicrobial Textile Finishes. Trends Textile Eng. Fashion Technol. 2018, 3, 4. [Google Scholar] [CrossRef]
- Sfameni, S.; Lawnick, T.; Rando, G.; Visco, A.; Textor, T.; Plutino, M.R. Super-Hydrophobicity of Polyester Fabrics Driven by Functional Sustainable Fluorine-Free Silane-Based Coatings. Gels 2023, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, M.; Pan, F.; Ning, X.; Ming, J. Influence of fluorescent dyes for dyeing of regenerated cellulose fabric. Text. Res. J. 2019, 90, 1385–1395. [Google Scholar] [CrossRef]
- Pishgar, M.; Gharanjig, K.; Yazdanshenas, M.E.; Farizadeh, K.; Rashidi, A.S. Synthesis and characterization of novel fluorescent reactive dyes for dyeing of cotton fabrics. J. Text. Inst. 2022, 11, 595–605. [Google Scholar] [CrossRef]
- Islam, K.; Kabir, S.M.; Hosen, D.; Islam, A. Fastness properties improvement of fluorescent pigments. Fibres Text. 2022, 29, 45–53. [Google Scholar] [CrossRef]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Purcar, V.; Ispas, G.; Frone, A.N.; Gabor, R.A.; Nicolae, C.-A. Optical Behavior of Curcuminoid Hybrid Systems as Coatings Deposited on Polyester Fibers. Coatings 2022, 12, 271. [Google Scholar] [CrossRef]
- Hussain, A.; Calabria-Holley, J.; Jiang, Y.; Lawrence, M. Modification of hemp shiv properties using water-repellent sol–gel coatings. J. Sol-Gel Sci. Technol. 2018, 86, 187–197. [Google Scholar] [CrossRef]
- Bakar, N.H.A.; Yusop, H.M.; Ismail, W.N.W.; Zulkifli, N.F. Sol-Gel Finishing for Protective Fabrics. Biointerface Res. Appl. Chem. 2023, 13, 283. [Google Scholar] [CrossRef]
- Raditoiu, A.; Amariutei, V.; Raditoiu, V.; Ghiurea, M.; Nicolae, C.A.; Wagner, L.E. Disperse Azo Dyes-silica Hybrids for Modifying Cellulose Fabrics by Sol-gel Processes. Rev. Chim. 2012, 63, 612–617. [Google Scholar]
- Raditoiu, A.; Amariutei, V.; Raditoiu, V.; Fierascu, R.C.; Nicolae, C.A.; Wagner, L.E. Non-ionic Dyes in Silica Hybrid Materials as Coloured Coatings on Protein Fibers. Rev. Chim. 2014, 65, 1002–1007. [Google Scholar]
- Kale, R.D.; Agnihotri, A.; Jagtap, P.S. Simultaneous dyeing and anti-bacterial finishing of textile by sol-gel technique. Adv. Appl. Sci. Res. 2016, 7, 116–122. [Google Scholar]
- Saha, J.; Chakraborty, S.S.; Rahman, A.; Sakif, R. Antimicrobial Activity of Textiles from Different Natural Resources. J. Res. Environ. Earth Sci. 2021, 7, 32–44. [Google Scholar]
- Arora, L.; Kaur, R. A Study on Antimicrobial Finish on Textile Using Plant Extract. Int. J. Innov. Res. Comput. Sci. Technol. 2022, 10, 2. [Google Scholar]
- Ennaceur, S.; Bouaziz, A.; Gargoubi, S.; Mnif, W.; Dridi, D. Enhanced Natural Dyeing and Antibacterial Properties of Cotton by Physical and Chemical Pretreatments. Processes 2022, 10, 2263. [Google Scholar] [CrossRef]
- Bouaziz, A.; Dridi, D.; Gargoubi, S.; Chelbi, S.; Boudokhane, C.; Kenani, A.; Aroui, S. Analysis of the Coloring and Antibacterial Effects of Natural Dye: Pomegranate Peel. Coatings 2021, 11, 277. [Google Scholar] [CrossRef]
- Bhuyan, S.; Gogoi, N.; Kalita, B.B. Natural dyes and its Antimicrobial effect. Int. J. Eng. Trends Technol. 2016, 42, 102–105. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Santos, G.; Marques, R.; Silva, S.; Oliveira, J.; Castro, P.; Pereira, C.; Pinheiro, M. Innovative High-Visibility Protective Clothing Development. Textiles 2021, 1, 405–418. [Google Scholar] [CrossRef]
- Xun, H.N.; Sheng, Z.; Tingting, C.; Xu, Y.F. Recent studies on cellulose-based fluorescent smart materials and their applications: A comprehensive review. Carbohydr. Polym. 2021, 267, 118135. [Google Scholar] [CrossRef]
- Geethadevi, C.; Rajendran, R.; Radhai, R.; Subraminam, D. Antimicrobial Efficacy of Nanoparticles Loaded with Herbal Extracts on Polyester/Cotton Blend Fabrics in Combating Nosocomial Infection. J. Hosp. Infect. 2019, 1, 1. [Google Scholar]
- Vukušić, S.B.; Grgac, S.F.; Budimir, A.; Kalenić, S. Cotton textiles modified with citric acid as efficient antibacterial agent for prevention of nosocomial infections. Croat. Med. J. 2011, 52, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Nicoloro, J.M.; Wen, J.; Queiroz, S.; Sun, Y.; Goodyear, N. A Novel Comprehensive Efficacy Test for Textiles Intended for Use in the Healthcare Setting. J. Microbiol. Methods 2020, 173, 105937. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K. The role of textiles as fomites in the healthcare environment: A review of the infection control risk. PeerJ 2020, 8, 9790. [Google Scholar] [CrossRef] [PubMed]
- Gotmare, V.D.; Kole, S.S.; Athawale, R.B. Sustainable approach for development of antimicrobial textile material using nanoemulsion for wound care applications. Fash. Text. 2018, 5, 25. [Google Scholar] [CrossRef]
- Schneider, G.; Bim, F.L.; Sousa, A.F.L.; Watanabe, E.; Andrade, D.; Fronteira, I. The use of antimicrobial-impregnated fabrics in health services: An integrative review. Rev. Lat.-Am. Enferm. 2021, 29, 3416. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial textile: Recent developments and functional perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef]
- Gao, Y.; Cranston, R. Recent Advances in Antimicrobial Treatments of Textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar] [CrossRef]
- Kamel, M.Y.; Hassabo, A.G. Anti-Microbial Finishing for Natural Textile Fabrics. J. Text. Color. Polym. Sci. 2021, 18, 83–95. [Google Scholar] [CrossRef]
- Elkashouty, M.; Elsyaed, H.; Twaffiek, S.; Salem, T.; Elhadad, S.S.M. An Overview: Textile Surface Modification by Using Sol-gel Technology. Egypt. J. Chem. 2020, 63, 3301–3311. [Google Scholar] [CrossRef]
- Colleoni, C.; Donelli, I.; Freddi, G.; Guido, E.; Migani, V.; Rosace, G. A novel sol-gel multi-layer approach for cotton fabric finishing by tetraethoxysilane precursor. Surf. Coat. Technol. 2013, 235, 192–203. [Google Scholar] [CrossRef]
- Vijay, K.; Nitesh, K.; Yashawant, R.; Ankur, S. Copper-Coated Uniform for Health Care Professional Could Help Reduce Cross Infection in Hospitals. J. Text. Sci. Eng. 2021, 11, 429. [Google Scholar]
- Mohamed, F.A.; Ibrahim, H.M. Antimicrobial dyes based on heterocyclic and/or homocyclic systems for dyeing and textile finishing. Res. Rev. Biosci. 2014, 8, 285–301. [Google Scholar]
- Gao, S.; Su, J.; Wang, W.; Fu, J.; Wang, H. Highly efficient and durable antibacterial cotton fabrics finished with zwitterionic polysulfobetaine by one-step ecofriendly strategy. Cellulose 2021, 28, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Veselova, V.O.; Plyuta, V.A.; Kostrov, A.N.; Vtyurina, D.N.; Abramov, V.O.; Abramova, A.V.; Voitov, Y.I.; Padiy, D.A.; Thu, V.T.H.; Hue, L.T.; et al. Long-Term Antimicrobial Performance of Textiles Coated with ZnO and TiO2 Nanoparticles in a Tropical Climate. J. Funct. Biomater. 2022, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Feng, L.; Xu, S.; Zhu, C.; Pan, G.; Yao, L. Universal Preparation Strategy for Ultradurable Antibacterial Fabrics through Coating an Adhesive Nanosilver Glue. Nanomaterials 2022, 12, 2429. [Google Scholar] [CrossRef] [PubMed]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Frone, A.N.; Nicolae, C.A.; Purcar, V.; Ispas, G.; Constantin, M.; Raut, I. Modeling the Properties of Curcumin Derivatives in Relation to the Architecture of the Siloxane Host Matrices. Materials 2022, 15, 267. [Google Scholar] [CrossRef]
- He, Z.; Nam, S.; Fang, D.D.; Cheng, H.N.; He, J. Surface and Thermal Characterization of Cotton Fibers of Phenotypes Differing in Fiber Length. Polymers 2021, 13, 994. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y. Fourier Transform Infrared Spectroscopic Analysis in Applied Cotton Fiber and Cottonseed Research: A Review. J. Cotton Sci. 2021, 25, 167–183. [Google Scholar] [CrossRef]
- Allen, A.; Foulk, J.; Gamble, G. Preliminary Fourier-Transform Infrared Spectroscopy Analysis of Cotton Trash. J. Cotton Sci. 2007, 11, 68–74. [Google Scholar]
- Yin, Y.; Yin, H.; Wu, Z.; Qi, C.; Tian, H.; Zhang, W.; Hu, Z.; Feng, L. Characterization of coals and coal ashes with high Si content using combined second-derivative infrared spectroscopy and Raman spectroscopy. Crystals 2019, 9, 513. [Google Scholar] [CrossRef]
- Hofmeister, A.M.; Bowey, J.E. Quantitative infrared spectra of hydrosilicates and related minerals. Mon. Not. R. Astron. Soc. 2006, 367, 577–591. [Google Scholar] [CrossRef]
- Sfameni, S.; Lawnick, T.; Rando, G.; Visco, A.; Textor, T.; Plutino, M.R. Functional Silane-Based Nanohybrid Materials for the Development of Hydrophobic and Water-Based Stain Resistant Cotton Fabrics Coatings. Nanomaterials 2022, 12, 3404. [Google Scholar] [CrossRef] [PubMed]
- Supharoek, S.; Ponhong, K.; Siriangkhawut, W.; Grudpan, K. Employing natural reagents from turmeric and lime for acetic acid determination in vinegar sample. J. Food Drug Anal. 2018, 26, 583–590. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Tan, S.; Tan, G.; Zhang, H.; Xia, N.; Jiang, L.; Ren, H.; Rayan, A.M. Intelligent colorimetric soy protein islate-based films incorporated with curcumin through an organic solvent-free pH-driven method: Properties, molecular interactions, and application. Food Hydrocoll. 2022, 133, 107904. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.W. Curcumin and its uses in active and smart food packaging applications—A comprehensive review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef]
- Patra, D. Christelle Barakat. Synchronous fluorescence spectroscopic study of solvatochromic curcumin dye. Spectrochim. Acta Part A 2011, 79, 1034–1041. [Google Scholar] [CrossRef]
- Raduly, M.F.; Raditoiu, V.; Raditoiu, A.; Wagner, L.E.; Amariutei, V.; Ailiesei Darvaru, G. Facile synthesis of curcumin and curcuminoid-like derivatives at microwaves. Rev. Chim. 2018, 69, 1327–1331. [Google Scholar] [CrossRef]
- Sharifian, P.; Yaslianifard, S.; Fallah, P.; Aynesazi, S.; Bakhtiyari, M.; Mohammadzadeh, M. Investigating the Effect of Nano-Curcumin on the Expression of Biofilm Regulatory Genes of Pseudomonas aeruginosa. Infect. Drug Resist. 2020, 13, 2477–2484. [Google Scholar] [CrossRef]
- Górski, M.; Niedźwiadek, J.; Magryś, A. Antibacterial activity of curcumin—A natural phenylpropanoid dimer from the rhizomes of Curcuma longa L. and its synergy with antibiotics. Ann. Agric. Environ. Med. 2022, 29, 394–400. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef] [PubMed]
- Gutarowska, B.; Machnowski, W.; Kowzowicz, L. Antimicrobial Activity of Textiles with Selected Dyes and Finishing Agents Used in the Textile Industry. Fibers Polym. 2013, 14, 415–422. [Google Scholar] [CrossRef]
- Pinho, E.; Magalhães, L.; Henriques, M.; Oliveira, R. Antimicrobial activity assessment of textiles: Standard methods comparison. Ann. Microbiol. 2011, 61, 493–498. [Google Scholar] [CrossRef]
- ISO 20645:2004; Textile Fabrics—Determination of Antibacterial Activity—Agar Diffusion Plate Test. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 105-C06; ISO Textiles—Test for Color Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 105-X12; ISO Textiles—Tests for Colour Fastness—Part X12: Colour Fastness to Rubbing. International Organization for Standardization: Geneva, Switzerland, 2016.









| Sample | SBET (m2·g−1) | Vtot (cm3·g−1) | DBJH (nm) |
|---|---|---|---|
| Cotton | 4.37 | 0.0056 | 2.97 |
| CY1 | 2.28 | 0.0032 | 4.26 |
| CY2 | 9.29 | 0.0091 | 3.45 |
| CR1 | 3.58 | 0.0013 | 3.31 |
| CR2 | 8.60 | 0.0050 | 2.99 |
| Sample | Abs. (λmax, nm) | K/S (a.u.) | K/S (a.u) Wash 1 | K/S (a.u.) Wash 5 | K/S (a.u.) Wash 15 |
|---|---|---|---|---|---|
| CY1 | 0.7109 (422) | 1.6668 | 1.5413 | 1.0610 | 1.0136 |
| CY2 | 0.7462 (396) | 1.8772 | 1.7476 | 1.0702 | 0.9453 |
| CR1 | 0.7513 (440) | 1.9087 | 1.4795 | 1.3469 | 1.2444 |
| CR2 | 0.7694 (434) | 2.0254 | 1.8306 | 1.3641 | 1.0893 |
| Sample | Washing Fastness (Grade) ISO 105 C06 | Rubbing Fastness (Grade) ISO 105 X12 | |||
|---|---|---|---|---|---|
| Color Change | Color Staining | ||||
| Cotton | Wool | Dry | Wet | ||
| CY1 | 4–5 | 4–5 | 4–5 | 4 | 3 |
| CY2 | 3 | 3–4 | 4 | 3–4 | 2–3 |
| CR1 | 4–5 | 4 | 4–5 | 3–4 | 2–3 |
| CR2 | 3–4 | 3–4 | 4 | 3–4 | 2–3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raduly, F.M.; Rădițoiu, V.; Rădițoiu, A.; Frone, A.N.; Nicolae, C.A.; Răut, I.; Constantin, M.; Grapin, M. Multifunctional Finishing of Cotton Fabric with Curcumin Derivatives Coatings Obtained by Sol–Gel Method. Gels 2023, 9, 369. https://doi.org/10.3390/gels9050369
Raduly FM, Rădițoiu V, Rădițoiu A, Frone AN, Nicolae CA, Răut I, Constantin M, Grapin M. Multifunctional Finishing of Cotton Fabric with Curcumin Derivatives Coatings Obtained by Sol–Gel Method. Gels. 2023; 9(5):369. https://doi.org/10.3390/gels9050369
Chicago/Turabian StyleRaduly, Florentina Monica, Valentin Rădițoiu, Alina Rădițoiu, Adriana Nicoleta Frone, Cristian Andi Nicolae, Iuliana Răut, Mariana Constantin, and Maria Grapin. 2023. "Multifunctional Finishing of Cotton Fabric with Curcumin Derivatives Coatings Obtained by Sol–Gel Method" Gels 9, no. 5: 369. https://doi.org/10.3390/gels9050369