Synthesis and Characterization of a Novel Composite Edible Film Based on Hydroxypropyl Methyl Cellulose Grafted with Gelatin
Abstract
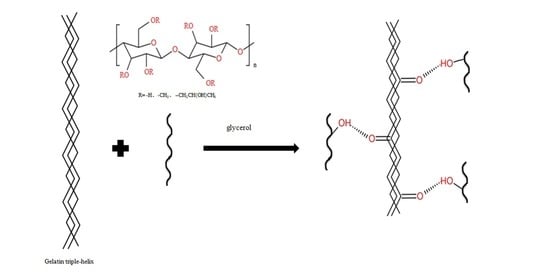
:1. Introduction
2. Results and Discussion
2.1. DSC
2.2. TGA
2.3. XRD Analysis
2.4. FT-IR of the Films
2.5. Mechanical Properties
2.6. SEM
2.7. Water Contact Angle
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the HPMC/Gel Composite Material
4.3. X-ray Diffraction Measurements
4.4. Fourier Transform Infrared Spectroscopy
4.5. Thermogravimetric Analysis
4.6. Differential Scanning Calorimetry
4.7. Mechanical Properties
4.8. Scanning Electron Microscopy
4.9. Water Contact Angle Tests
4.10. Statistic Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arfat, Y.A.; Ejaz, M.; Jacob, H.; Ahmed, J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohyd. Polym. 2017, 157, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Giannakas, A.E.; Moschovas, D.; Kollia, E.; Georgopoulos, S.; Gioti, C.; Leontiou, A.; Avgeropoulos, A.; Kopsacheili, A.; Avdylaj, L.; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels 2022, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Han, X.S.; Zhang, C.M.; Liu, K.M.; Duan, G.G. Source of Nanocellulose and its application in nanocomposite packaging Material: A Review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, M.; Li, G. Preparation and characterization of collagen/hydroxypropyl methylcellulose (HPMC) blend film. Carbohyd. Polym. 2015, 119, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Elfahmy, S.; Revoljunelles-Junelles, A.M.; Desobry, S. Cellulose derivative based active coatings: Effects of nisin and plasticizer on physico-chemical and antimicrobial properties of hydroxypropyl methylcellulose films. Carbohyd. Polym. 2010, 81, 219–225. [Google Scholar] [CrossRef]
- Villalobos, R.; Chanona, J.; Hernández, P.; Gutiérrez, G.; Chiralt, A. Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocoll. 2005, 19, 53–61. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Klangmuang, P.; Sothornvit, R. Barrier properties, mechanical properties and antimicrobial activity of hydroxypropyl methylcellulose-based nanocomposite films incorporated with Thai essential oils. Food Hydrocoll. 2016, 61, 609–616. [Google Scholar] [CrossRef]
- Liang, Z.; Long, Y.; Liu, H.; Wang, Y.; Simon, G.P.; Ji, Z.; Qian, J. Effect of processing conditions on microstructures and properties of hydroxypropyl methylcellulose/hydroxypropyl starch blends. Food Hydrocoll. 2017, 70, 251–259. [Google Scholar] [CrossRef]
- Kołodziejska, I.; Sikorski, Z.E.; Niecikowska, C. Parameters affecting the isolation of collagen from squid (Illexargentinus) skins. Food Chem. 1999, 66, 153–157. [Google Scholar] [CrossRef]
- Collins, R.L.; Christiansen, D.; Zazanis, G.A.; Silver, F.H. Use of collagen film as a dural substitute: Preliminary animal studies. J. Biomed. Mater. Res. 2010, 25, 267–276. [Google Scholar] [CrossRef]
- Maeda, M.; Kadota, K.; Kajihara, M.; Sano, A.; Fujioka, K. Sustained release of human growth hormone (hGH) from collagen film and evaluation of effect on wound healing in db/db mice. J. Control. Release 2001, 77, 261–272. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Tech. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Alberto, J.; María José, F.; Amparo, C. Influence of hydroxypropylmethylcellulose addition and homogenization conditions on properties and ageing of corn starch based films. Carbohyd. Polym. 2012, 89, 676–686. [Google Scholar] [CrossRef]
- Aydogdu, A.; Yildiz, E.; Ayhan, Z.; Aydogdu, Y.; Sahin, S. Nanostructured Poly(lactic acid)/Soy Protein/HPMC films by electrospinning for potential applications in food industry. Eur. Polym. J. 2019, 112, 477–486. [Google Scholar] [CrossRef]
- Basch, C.Y.; Jagus, R.J.; Flores, S.K. Physical and Antimicrobial Properties of Tapioca Starch-HPMC Edible Films Incorporated with Nisin and/or Potassium Sorbate. Food Bioprocess Tech. 2013, 6, 2419–2428. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Properties of starch–hydroxypropyl methylcellulose based films obtained by compression molding. Carbohyd. Polym. 2014, 109, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. A novel electrospun hydroxypropyl methylcellulose/polyethylene oxide blend nanofibers: Morphology and physicochemical properties. Carbohyd. Polym. 2018, 181, 234–246. [Google Scholar] [CrossRef]
- Kriegel, C.; Kit, K.M.; Mcclements, D.J.; Weiss, J. Influence of Surfactant Type and Concentration on Electrospinning of Chitosan–Poly(Ethylene Oxide) Blend Nanofibers. Food Biophys. 2009, 4, 213–228. [Google Scholar] [CrossRef]
- Fei, L.; Majeed, H.; Antoniou, J.; Yue, L.; Fang, Z. Tailoring physical properties of transglutaminase-modified gelatin films by varying drying temperature. Food Hydrocoll. 2016, 58, 20–28. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Structural, morphological and thermal behaviourcharacterisations of fish gelatin film incorporated with basil and citronella essential oils as affected by surfactants. Food Hydrocoll. 2014, 41, 33–43. [Google Scholar] [CrossRef]
- Shen, Y.T.; Tang, X.; Li, Y.H. Drying methods affect physicochemical and functional properties of quinoa protein isolate. Food Chem. 2021, 339, 127823. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Luo, K.; Chen, X.; Khutoryanskiy, V.V. Miscibility studies of the blends of chitosan with some cellulose ethers. Carbohyd. Polym. 2006, 63, 238–244. [Google Scholar] [CrossRef]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun Silk Fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef]
- Rujitanaroj, P.O.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Rani, N.; Sannappa, J.; Demappa, T.; Mahadevaiah, T. Effects of CdCl 2 concentration on the structural, thermal and ionic conductivity properties of HPMC polymer electrolyte films. Ionics 2015, 21, 133–140. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.F.; Liu, H.; Yu, L.; Wang, Y.; Simon, G.P.; Qian, J. Effect of plasticizers on microstructure, compatibility and mechanical property of hydroxypropyl methylcellulose/hydroxypropyl starch blends. Int. J. Biol. Macromol. 2018, 119, 141–148. [Google Scholar] [CrossRef]
- Badii, F.; Macnaughtan, W.; Mitchell, J.R.; Farhat, I.A. The Effect of Drying Temperature on Physical Properties of Thin Gelatin Films. Dry. Technol. 2014, 32, 30–38. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohyd. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Priya, D.S.; Suriyaprabha, R.; Yuvakkumar, R.; Rajendran, V. Chitosan-incorporated different nanocomposite HPMC films for food preservation. J. Nanopart. Res. 2014, 16, 2248. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, H.; Zheng, H.; Xu, Y.; Zhang, C. Structure and properties of blend fibers prepared from alginate and konjac glucomannan. J. App. Polym. Sci. 2010, 106, 3903–3907. [Google Scholar] [CrossRef]
- Techawinyutham, L.; Tengsuthiwat, J.; Srisuk, R.; Techawinyutham, W.; Rangappa, S.M.; Siengchin, S. Recycled LDPE/PETG blends and HDPE/PETG blends: Mechanical, thermal, and rheological properties. J. Mater. Res. Technol. 2021, 15, 2445–2458. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Liu, H.; Yu, L.; Simon, G.P.; Zhang, N.; Chen, L. Relationship between morphologies and mechanical properties of hydroxypropyl methylcellulose/hydroxypropyl starch blends. Carbohyd. Polym. 2016, 153, 329–335. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Wang, P.; He, H.; Cai, R.; Tao, G.; Yang, M.; Zuo, H.; Wang, Y. Cross-linking of dialdehyde carboxymethyl cellulose with silk sericin to reinforce sericin film for potential biomedical application. Carbohyd. Polym. 2019, 212, 403–411. [Google Scholar] [CrossRef]
- Hosen, M.D.; Hossain, M.S.; Islam, M.A.; Haque, A.N.M.A.; Naebe, M. Utilisation of natural wastes: Water-resistant semi-transparent paper for food packaging. J. Clean. Prod. 2022, 364, 132665. [Google Scholar] [CrossRef]






| Sample No. | HPMC/Gel | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| 1 | 10/0 | 26.08 ± 1.51 | 23.2 ± 2.1 |
| 2 | 8/2 | 28.05 ± 0.99 | 21.7 ± 3.2 |
| 3 | 6/4 | 29.58 ± 0.36 | 18.9 ± 1.4 |
| 4 | 5/5 | 32.93 ± 1.37 | 16.9 ± 2.3 |
| 5 | 4/6 | 26.76 ± 0.74 | 16.2 ± 2.2 |
| 6 | 2/8 | 33.42 ± 1.29 | 16.1 ± 2.1 |
| 7 | 0/10 | 42.69 ± 1.36 | 9.4 ± 1.2 |
| HPMC/Gel | WCA (°) (Mean ± SD) |
|---|---|
| 10/0 | 52.01 ± 0.63 |
| 8/2 | 61.22 ± 0.99 |
| 6/4 | 67.05 ± 0.14 |
| 5/5 | 85.13 ± 0.56 |
| 4/6 | 86.41 ± 0.90 |
| 2/8 | 92.46 ± 0.20 |
| 0/10 | 101.23 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jiang, S.; Chen, Y.; Qiu, D.; Weng, Y. Synthesis and Characterization of a Novel Composite Edible Film Based on Hydroxypropyl Methyl Cellulose Grafted with Gelatin. Gels 2023, 9, 332. https://doi.org/10.3390/gels9040332
Wang Y, Jiang S, Chen Y, Qiu D, Weng Y. Synthesis and Characterization of a Novel Composite Edible Film Based on Hydroxypropyl Methyl Cellulose Grafted with Gelatin. Gels. 2023; 9(4):332. https://doi.org/10.3390/gels9040332
Chicago/Turabian StyleWang, Yajuan, Shuting Jiang, Yue Chen, Dan Qiu, and Yunxuan Weng. 2023. "Synthesis and Characterization of a Novel Composite Edible Film Based on Hydroxypropyl Methyl Cellulose Grafted with Gelatin" Gels 9, no. 4: 332. https://doi.org/10.3390/gels9040332