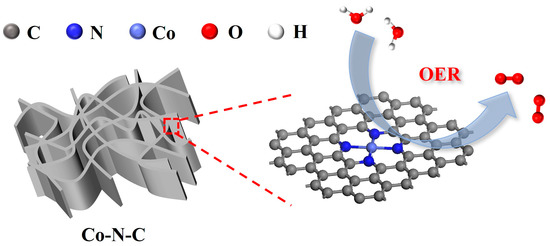
Tuning the Electronic Structure of a Novel 3D Architectured Co-N-C Aerogel to Enhance Oxygen Evolution Reaction Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition and Structural Analysis
2.2. OER Performance Analysis
2.3. Theoretical Calculations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Method
4.3. Characterizations
4.4. Electrochemical Measurements
4.5. Theoretical Calculations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Fang, Z.; Li, H.; Fernandez, D.; Henkelman, G.; Humphrey, S.M.; Yu, G. Rational Design of Rhodium-Iridium Alloy Nanoparticles as Highly Active Catalysts for Acidic Oxygen Evolution. ACS Nano 2019, 13, 13225–13234. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.C.; Fernandes, M.R.; Ticianelli, E.A. Activity and Stability of Pt/IrO2 Bifunctional Materials as Catalysts for the Oxygen Evolution/Reduction Reactions. ACS Catal. 2018, 8, 2081–2092. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Jiang, P.; Yang, K.; Diao, J.; Gong, S.; Liu, S.; Huang, M.; Wang, H.; Chen, Q. Mn-Doped RuO2 Nanocrystals as Highly Active Electrocatalysts for Enhanced Oxygen Evolution in Acidic Media. ACS Catal. 2020, 10, 1152–1160. [Google Scholar] [CrossRef]
- Han, L.; Dong, S.; Wang, E. Transition-Metal (Co, Ni, and Fe)-Based Electrocatalysts for the Water Oxidation Reaction. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar] [CrossRef]
- Hu, C.; Paul, R.; Dai, Q.; Dai, L. Carbon-based metal-free electrocatalysts: From oxygen reduction to multifunctional electrocatalysis. Chem. Soc. Rev. 2021, 50, 11785–11843. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xiao, J.; Wang, H.Y.; Shao, M.H. Carbon-Based Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. ACS Catal. 2017, 7, 7855–7865. [Google Scholar] [CrossRef]
- Qiu, H.J.; Johnson, I.; Chen, L.; Cong, W.; Ito, Y.; Liu, P.; Han, J.; Fujita, T.; Hirata, A.; Chen, M. Graphene-coated nanoporous nickel towards a metal-catalyzed oxygen evolution reaction. Nanoscale 2021, 13, 10916–10924. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Du, Y.; Huang, S.; Mollamahale, B.Y.; Zhang, X.; Hasan, S.W.; Yu, F.; Wang, S.; Tian, Z.Q.; Shen, P.K. Highly Efficient Multifunctional Co N C Electrocatalysts with Synergistic Effects of Co N Moieties and Co Metallic Nanoparticles Encapsulated in a N -Doped Carbon Matrix for Water -Splitting and Oxygen Redox Reactions. ACS Appl. Mater. Interfaces 2019, 11, 39809–39819. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Wang, X.Y.; Li, Z.J.; Yang, B.; Ling, M.; Gao, X.; Lu, J.G.; Shi, Q.R.; Lei, L.C.; Wu, G.; et al. Designing 3d dual transition metal electrocatalysts for oxygen evolution reaction in alkaline electrolyte: Beyond oxides. Nano Energy 2020, 77, 105162. [Google Scholar] [CrossRef]
- Cai, B.; Eychmuller, A. Promoting Electrocatalysis upon Aerogels. Adv. Mater. 2019, 31, 1804881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.T.; Tai, J.X.; Hao, W.K.; Lu, J.X.; Liu, S.J.; Wu, X.D.; Cui, S. A novel magnetic ZnFe2O4 aerogel photocatalyst for visible light reduction of Cr(VI) at high concentrations: Facile synthesis, enhanced activity and photocatalytic mechanism, combined with first-principles calculations. Ceram. Int. 2022, 48, 3974–3984. [Google Scholar] [CrossRef]
- Yan, W.Q.; Zhu, K.M.; Cui, Y.; Li, Y.H.; Dai, T.; Cui, S.; Shen, X.D. NO2 detection and redox capacitance reaction of Ag doped SnO2/rGO aerogel at room temperature. J. Alloys Compd. 2021, 886, 161287. [Google Scholar] [CrossRef]
- Bai, X.J.; Lu, X.Y.; Ju, R.; Chen, H.; Shao, L.; Zhai, X.; Li, Y.N.; Fan, F.Q.; Fu, Y.; Qi, W. Preparation of MOF Film/Aerogel Composite Catalysts via Substrate-Seeding Secondary-Growth for the Oxygen Evolution Reaction and CO2 Cycloaddition. Angew. Chem. Int. Edit. 2021, 60, 701–705. [Google Scholar] [CrossRef]
- Fu, G.T.; Yan, X.X.; Chen, Y.F.; Xu, L.; Sun, D.M.; Lee, J.M.; Tang, Y.W. Boosting bifunctional oxygen electrocatalysis with 3D graphene aerogel-supported Ni/MnO particles. Adv. Mater. 2018, 30, 1704609. [Google Scholar] [CrossRef]
- Cui, C.; Lai, X.X.; Guo, R.H.; Ren, E.H.; Qin, W.F.; Liu, L.; Zhou, M.; Xiao, H.Y. Waste paper-based carbon aerogel supported ZIF-67 derived hollow NiCo phosphate nanocages for electrocatalytic oxygen evolution reaction. Electrochim. Acta 2021, 393, 139076. [Google Scholar] [CrossRef]
- Yan, S.; Zhong, M.X.; Wang, C.; Lu, X.F. Amorphous aerogel of trimetallic FeCoNi alloy for highly efficient oxygen evolution. Chem. Eng. J. 2022, 430, 132955. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, S.; Ren, J.; Jia, Y.A.; Chen, C.M.; Komarneni, S.; Yang, D.J.; Yao, X.D. Electronic Structure Tuning in Ni3FeN/r-GO Aerogel toward Bifunctional Electrocatalyst for Overall Water Splitting. ACS Nano 2018, 12, 245–253. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Lu, R.Q.; Sam, D.K.; Wang, W.B.; Gong, S.H.; Liu, J.; Durairaj, A.; Li, M.X.; Lv, X.M. Boron, nitrogen co-doped biomass-derived carbon aerogel embedded nickel-cobalt-iron nanoparticles as a promising electrocatalyst for oxygen evolution reaction. J. Colloid Interf Sci. 2022, 613, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Zhang, X.F.; Li, Y.T.; Wang, J.; Kawi, S.; Zhong, Q. FeCo alloy/N, S co-doped carbon aerogel derived from directional-casting cellulose nanofibers for rechargeable liquid flow and flexible Zn-air batteries. Nano Res. 2023, 1, 193. [Google Scholar] [CrossRef]
- Fei, H.L.; Dong, J.C.; Feng, Y.X.; Allen, C.S.; Wan, C.Z.; Volosskiy, B.; Li, M.F.; Zhao, Z.P.; Wang, Y.L.; Sun, H.T.; et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Lin, L.C.; Grossman, J.C. Atomistic understandings of reduced graphene oxide as an ultrathin-film nanoporous membrane for separations. Nat. Commun. 2015, 6, 8335. [Google Scholar] [CrossRef] [Green Version]
- Tsang, C.H.A.; Hui, K.N.; Hui, K.S.; Ren, L. Deposition of Pd/graphene aerogel on nickel foam as a binder-free electrode for direct electro-oxidation of methanol and ethanol. J. Mater. Chem. A 2014, 2, 17986–17993. [Google Scholar] [CrossRef]
- Razmjooei, F.; Singh, K.P.; Yang, D.S.; Cui, W.; Jang, Y.H.; Yu, J.S. Fe-Treated Heteroatom (S/N/B/P)-Doped Graphene Electrocatalysts for Water Oxidation. ACS Catal. 2017, 7, 2381–2391. [Google Scholar] [CrossRef]
- Xu, L.M.; Zhang, Y.Y.; Zhou, W.Q.; Jiang, F.X.; Zhang, H.; Jiang, Q.L.; Jia, Y.H.; Wang, R.; Liang, A.Q.; Xu, J.K.; et al. Fused Heterocyclic Molecule-Functionalized N-Doped Reduced Graphene Oxide by Non-Covalent Bonds for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 45202–45213. [Google Scholar] [CrossRef]
- Gupta, S.; Qiao, L.; Zhao, S.; Xu, H.; Lin, Y.; Devaguptapu, S.V.; Wang, X.L.; Swihart, M.T.; Wu, G. Highly Active and Stable Graphene Tubes Decorated with FeCoNi Alloy Nanoparticles via a Template-Free Graphitization for Bifunctional Oxygen Reduction and Evolution. Adv. Energy Mater. 2016, 6, 1601198. [Google Scholar] [CrossRef]
- He, D.P.; Xiong, Y.L.; Yang, J.L.; Chen, X.; Deng, Z.X.; Pan, M.; Li, Y.D.; Mu, S.C. Nanocarbon-intercalated and Fe-N-codoped graphene as a highly active noble-metal-free bifunctional electrocatalyst for oxygen reduction and evolution. J. Mater. Chem. A. 2017, 5, 1930–1934. [Google Scholar] [CrossRef]
- Zou, H.Y.; Li, G.; Duan, L.L.; Kou, Z.K.; Wang, J. In situ coupled amorphous cobalt nitride with nitrogen-doped graphene aerogel as a trifunctional electrocatalyst towards Zn-air battery deriven full water splitting. Appl. Catal. B-Environ. 2019, 259, 118100. [Google Scholar] [CrossRef]
- Kang, Q.L.; Lai, D.W.; Su, M.F.; Xiong, B.R.; Tang, W.Y.; Lu, Q.Y.; Gao, F. Tailored dodecahedral polyoxometalates nanoframes with in situ encapsulated Co, N, C for oxygen evolution reaction. Chem. Eng. J. 2022, 430, 133116. [Google Scholar] [CrossRef]
- Liang, Z.Z.; Kong, N.N.; Yang, C.X.; Zhang, W.; Zheng, H.Q.; Lin, H.P.; Cao, R. Highly Curved Nanostructure-Coated Co, N-Doped Carbon Materials for Oxygen Electrocatalysis. Angew. Chem. Int. Edit. 2021, 60, 12759–12764. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.Q.; Gan, C.Q.; Wu, X.D.; Liu, Z.Z.; Tang, J.G. Facile synthesis of black phosphorus directly grown on carbon paper as an efficient OER Electrocatalyst: Role of Interfacial charge transfer and induced local charge distribution. Adv. Powder Technol. 2022, 33, 103371. [Google Scholar] [CrossRef]
- Xu, Z.H.; Fan, H.L.; Zhou, X.X.; Huang, G.P.; Wu, X.; Shen, M.R. Coating of Ni on Fe (oxy)hydroxide: Superior Catalytic Activity for Oxygen-Involved Reaction During Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 19832–19838. [Google Scholar] [CrossRef]
- Guan, J.Q.; Duan, Z.Y.; Zhang, F.X.; Kelly, S.D.; Si, R.; Dupuis, M.; Huang, Q.G.; Chen, J.Q.; Tang, C.H.; Li, C. Water oxidation on a mononuclear manganese heterogeneous catalyst. Nat. Catal. 2018, 1, 870–877. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, H.; Zheng, X.R.; Chen, Y.A.; Rogach, A.L.; Han, X.P.; Deng, Y.D.; Hu, W.B. Encapsulating Cobalt Nanoparticles in Interconnected N-Doped Hollow Carbon Nanofibers with Enriched Co-N-C Moiety for Enhanced Oxygen Electrocatalysis in Zn-Air Batteries. Adv. Sci. 2021, 8, 2101438. [Google Scholar] [CrossRef]
- Sam, D.K.; Gong, S.H.; Durairaj, A.; Sam, E.K.; Liu, J.; Lv, X.M. Fabrication of highly dispersed Mo2C coupled with Co-N-C via self-template as bifunctional electrocatalysts. Int. J. Energy Res. 2021, 45, 10989–11001. [Google Scholar] [CrossRef]
- Xu, G.; Xu, G.C.; Ban, J.J.; Zhang, L.; Lin, H.; Qi, C.L.; Sun, Z.P.; Jia, D.Z. Cobalt and cobalt oxides N-codoped porous carbon derived from metal-organic framework as bifunctional catalyst for oxygen reduction and oxygen evolution reactions. J. Colloid Interf Sci. 2018, 521, 141–149. [Google Scholar] [CrossRef]
- Wang, D.; Yang, P.X.; Xu, H.; Ma, J.Y.; Du, L.; Zhang, G.X.; Li, R.P.; Jiang, Z.; Li, Y.; Zhang, J.Q.; et al. The dual-nitrogen-source strategy to modulate a bifunctional hybrid Co/Co-N-C catalyst in the reversible air cathode for Zn-air batteries. J. Power Sources 2021, 485, 229339. [Google Scholar] [CrossRef]
- Wang, R.; Cao, J.Y.; Cai, S.C.; Yan, X.M.; Li, J.S.; Yourey, W.M.; Tong, W.; Tang, H.L. MOF@Cellulose Derived Co-N-C Nanowire Network as an Advanced Reversible Oxygen Electrocatalyst for Rechargeable Zinc-Air Batteries. ACS Appl. Energ. Mater. 2018, 1, 1060–1068. [Google Scholar] [CrossRef]
- Xin, W.L.; Lu, K.K.; Zhu, D.R.; Zeng, H.B.; Zhang, X.J.; Marks, R.S.; Shan, D. Highly reactive N,N’-carbonyldiimidazole-tailored bifunctional electrocatalyst for oxygen reduction and oxygen evolution. Electrochim. Acta 2019, 307, 375–384. [Google Scholar] [CrossRef]
- Xiang, F.; Yang, J.; Gong, W.X.; Zou, J.; Liu, Y.Z.; Li, Y.L.; Guo, H.; Wang, L.P.; Niu, X.B. Facile Synthesis of Graphene-like Porous Carbon with Densely Populated Co-N-x Sites as Efficient Bifunctional Electrocatalysts for Rechargeable Zinc-Air Batteries. ACS Appl. Energ. Mater. 2021, 4, 11545–11554. [Google Scholar] [CrossRef]
- Hao, Y.X.; Kang, Y.M.; Mi, Y.J.; Wang, W.; Lei, Z.Q. Highly ordered micro-meso-macroporous Co-N-doped carbon polyhedrons from bimetal-organic frameworks for rechargeable Zn-air batteries. J. Colloid Interf Sci. 2021, 598, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.C.; Qiu, L.J.; Liu, Y.Y.; Zhang, J.M.; Yuan, D.S.; Wang, L. Mn-Doped Co-N-C Dodecahedron as a Bifunctional Electrocatalyst for Highly Efficient Zn-Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 14180–14188. [Google Scholar] [CrossRef]
- Ding, Y.J.; Yang, W.Y.; Gao, S.; Sun, W.Z.; Sun, C.X.; Li, Q. Strongly Cooperative Nano-CoO/Co Active Phase in Hierarchically Porous Nitrogen-Doped Carbon Microspheres for Efficient Bifunctional Oxygen Electrocatalysis. ACS Appl. Energ. Mater. 2020, 3, 1328–1337. [Google Scholar] [CrossRef]
- Hu, E.L.; Ning, J.Q.; He, B.; Li, Z.P.; Zheng, C.C.; Zhong, Y.J.; Zhang, Z.Y.; Hu, Y. Unusual formation of tetragonal microstructures from nitrogen-doped carbon nanocapsules with cobalt nanocores as a bi-functional oxygen electrocatalyst. J. Mater. Chem. A. 2017, 5, 2271–2279. [Google Scholar] [CrossRef]
- Sarkar, S.; Biswas, A.; Siddharthan, E.E.; Thapa, R.; Dey, R.S. Strategic Modulation of Target-Specific Isolated Fe,Co Single-Atom Active Sites for Oxygen Electrocatalysis Impacting High Power Zn-Air Battery. ACS Nano 2022, 16, 7890–7903. [Google Scholar] [CrossRef]
- Tian, Y.H.; Xu, L.; Bao, J.; Qian, J.C.; Su, H.N.; Li, H.M.; Gu, H.D.; Yan, C.; Li, H.N. Hollow cobalt oxide nanoparticles embedded in nitrogen-doped carbon nanosheets as an efficient bifunctional catalyst for Zn-air battery. J. Energy Chem. 2019, 33, 59–66. [Google Scholar] [CrossRef]
- Shen, M.X.; Gao, K.; Xiang, F.K.; Wang, B.B.; Dai, L.; Zheng, L.Q.; Baker, F.; Duan, C.; Zhang, Y.H.; Sun, S.H.; et al. Nanocellulose-assisted synthesis of ultrafine Co nanoparticles-loaded bimodal micro-mesoporous N-rich carbon as bifunctional oxygen electrode for Zn-air batteries. J. Power Sources 2022, 450, 227640. [Google Scholar] [CrossRef]
- Pei, Z.X.; Huang, Y.; Tang, Z.J.; Ma, L.T.; Liu, Z.X.; Xue, Q.; Wang, Z.F.; Li, H.F.; Chen, Y.; Zhi, C.Y. Enabling highly efficient, flexible and rechargeable quasi-solid-state zn-air batteries via catalyst engineering and electrolyte functionalization. Energy Storage Mater. 2019, 20, 234–242. [Google Scholar] [CrossRef]







| Catalyst | Micropore Pore Volumes (cm3/g) | BET Specific Surface Areas (m2/g) | BJH Adsorption Average Pore Diameters (nm) | BJH Adsorption Pore Volumes (cm3/g) |
|---|---|---|---|---|
| GA | 0.12 | 69.73 | 6.81 | 0.11 |
| Co-C | 0.06 | 56.29 | 4.51 | 0.05 |
| Co-N-C | 0.04 | 42.93 | 3.50 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, C.; Huang, S.; Koudama, T.D.; Wu, X.; Cui, S.; Shen, X.; Chen, X. Tuning the Electronic Structure of a Novel 3D Architectured Co-N-C Aerogel to Enhance Oxygen Evolution Reaction Activity. Gels 2023, 9, 313. https://doi.org/10.3390/gels9040313
Ni C, Huang S, Koudama TD, Wu X, Cui S, Shen X, Chen X. Tuning the Electronic Structure of a Novel 3D Architectured Co-N-C Aerogel to Enhance Oxygen Evolution Reaction Activity. Gels. 2023; 9(4):313. https://doi.org/10.3390/gels9040313
Chicago/Turabian StyleNi, Chunsheng, Shuntian Huang, Tete Daniel Koudama, Xiaodong Wu, Sheng Cui, Xiaodong Shen, and Xiangbao Chen. 2023. "Tuning the Electronic Structure of a Novel 3D Architectured Co-N-C Aerogel to Enhance Oxygen Evolution Reaction Activity" Gels 9, no. 4: 313. https://doi.org/10.3390/gels9040313