Highly Efficient Capture of Heavy Metal Ions on Amine-Functionalized Porous Polymer Gels
Abstract
:1. Introduction
2. Results and Discussion
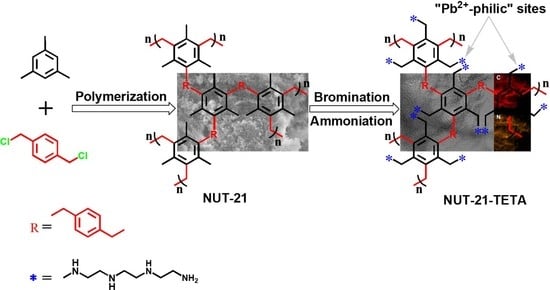
2.1. Structural and Surface Properties
2.2. Metal Ion Adsorption Performance
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Synthesis of Materials
4.3. Structural Characterization
4.4. Batch Adsorption Experiment
4.4.1. Adsorption Thermodynamics Test
4.4.2. Adsorption Kinetics Test
4.4.3. Various Metal Ions Adsorption Test
4.4.4. Recyclability Test
4.4.5. Breakthrough Experiment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, M.; Tashkandi, N.; Hadjichristidis, N.; Alkayal, N.S. Synthesis of naphthalene-based polyaminal-linked porous polymers for highly effective uptake of CO2 and heavy metals. Polymers 2022, 14, 1136. [Google Scholar] [CrossRef] [PubMed]
- Bakker, K. Water security: Research challenges and opportunities. Science 2012, 337, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Abd-Elhamid, A.I.; Awwad, N.S.; Abdelgawad, M.A.; Wu, J.; Mo, X.; Gomha, S.M.; Aly, A.A.; Bräse, S. Review of the recent advances in electrospun nanofibers applications in water purification. Polymers 2022, 14, 1594. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Aguila, B.; Perman, J.; Earl, L.D.; Abney, C.W.; Cheng, Y.; Wei, H.; Nguyen, N.; Wojtas, L.; Ma, S. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal. J. Am. Chem. Soc. 2017, 139, 2786–2793. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-based adsorbent material for the effective removal of heavy metals from wastewater: A comprehensive review. Gels 2022, 8, 263. [Google Scholar] [CrossRef]
- Burke, D.M.M.; Morris, M.A.; Holmes, J.D. Chemical oxidation of mesoporous carbon foams for lead ion adsorption. Sep. Purif. Technol. 2013, 104, 150–159. [Google Scholar] [CrossRef]
- Ahn, H.H.; Lee, M.S. Recoveries of Ru(III) and Co(II) by solvent extraction and ion exchange from tungsten carbide-cobalt scrap through a HCl leaching solution. Metals 2019, 9, 858. [Google Scholar] [CrossRef] [Green Version]
- Quiton, K.G.N.; Huang, Y.-H.; Lu, M.-C. Recovery of cobalt and copper from single- and co-contaminated simulated electroplating wastewater via carbonate and hydroxide precipitation. Sustain. Environ. Res. 2022, 32, 31. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhu, C.Y.; Lu, F.; Yu, Z.F.; Yang, H.C.; Xue, M.; Xu, Z.K. Metal-polyphenol coordination at the aqueous contra-diffusion “interface”: A green way to high-performance iron(iii)/tannic acid thin-film-composite nanofiltration membranes. Langmuir 2022, 38, 13793–13802. [Google Scholar] [CrossRef]
- Gong, C.; Ren, X.; Han, J.; Wu, Y.; Gou, Y.; Zhang, Z.; He, P. Toxicity reduction of reverse osmosis concentrates from petrochemical wastewater by electrocoagulation and Fered-Fenton treatments. Chemosphere 2022, 286, 131582. [Google Scholar] [CrossRef]
- Tang, X.; Hu, W.; Ke, X.; Zheng, Y.; Ge, Q. Antibacterial and desalting behavior of forward osmosis membranes engineered with metal ions. Desalination 2022, 530, 115655. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A.; Lindo, M.; Gascon, V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel adsorbents for the removal of hazardous pollutants—Requirements and available functions as adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Chapter 1—Fundamentals of adsorption technology. Interface Sci. Technol. 2021, 33, 1–70. [Google Scholar]
- Li, L.; Lin, R.-B.; Krishna, R.; Li, H.; Xiang, S.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Ethane-ethylene separation in a metal-organic framework with iron-peroxo sites. Science 2018, 362, 443–446. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Kong, L.; Yang, W.; Huang, Y.; Li, H.; Ma, S.; Lv, W.; Hu, J.; Wang, H.; Liu, H. Hooped amino-group chains in porous organic polymers for enhancing heavy metal ion removal. ACS Appl. Mater. Interface 2019, 11, 44751–44757. [Google Scholar] [CrossRef]
- Chen, Z.; Chan, A.K.; Wong, V.C.; Yam, V.W. A supramolecular strategy toward an efficient and selective capture of platinum(II) complexes. J. Am. Chem. Soc. 2019, 141, 11204–11211. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, M.A.B.H.; Carabineiro, S.A.C. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef]
- Peng, A.-Z.; Qi, S.-C.; Liu, X.; Xue, D.-M.; Peng, S.-S.; Yu, G.-X.; Liu, X.-Q.; Sun, L.-B. N-doped porous carbons derived from a polymer precursor with a record-high N content: Efficient adsorbents for CO2 capture. Chem. Eng. J. 2019, 372, 656–664. [Google Scholar] [CrossRef]
- Wang, L.-W.; Xia, B.-B.; Rao, L.-L.; Wang, L.-L.; Yue, L.-M.; Liang, Y.-Q.; DaCosta; Hu, X. Highly efficient CO2 adsorption by nitrogen-doped porous carbons synthesized with low-temperature sodium amide activation. Carbon 2018, 130, 31–40. [Google Scholar] [CrossRef]
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, aaa8075. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Lv, W.; Khan, I.; Liu, S.-Y. Photocatalytic H2 generation via CoP quantum-dot-modified g-C3N4 synthesized by electroless plating. Chin. J. Catal. 2020, 41, 114–121. [Google Scholar] [CrossRef]
- Qi, K.; Zhuang, C.; Zhang, M.; Gholami, P.; Khataee, A. Sonochemical synthesis of photocatalysts and their applications. J. Mater. Sci. Technol. 2022, 123, 243–256. [Google Scholar] [CrossRef]
- Gao, T.N.; Wang, T.; Wu, W.; Liu, Y.; Huo, Q.; Qiao, Z.A.; Dai, S. Solvent-induced self-assembly strategy to synthesize well-defined hierarchically porous polymers. Adv. Mater. 2019, 1806254, 1806254. [Google Scholar] [CrossRef] [PubMed]
- Tilford, R.W.; Mugavero, S.J.; Pellechia, P.J.; Lavigne, J.J. Tailoring microporosity in covalent organic frameworks. Adv. Mater. 2008, 20, 2741–2746. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.-M.; Rao, L.-L.; Wang, L.-W.; Wang, L.-L.; Wu, J.-Y.; Hu, X.; DaCosta, H.; Yang, J.; Fan, M.-H. Efficient CO2 capture by nitrogen-doped biocarbons derived from rotten strawberries. Ind. Eng. Chem. Res. 2017, 56, 14115–14122. [Google Scholar] [CrossRef]
- Yuan, M.; Yao, H.; Xie, L.; Liu, X.; Wang, H.; Islam, S.M.; Shi, K.; Yu, Z.; Sun, G.; Li, H.; et al. Polypyrrole-Mo3S13: An efficient sorbent for the capture of Hg(2+) and highly selective extraction of Ag(+) over Cu(2+). J. Am. Chem. Soc. 2020, 142, 1574–1583. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R.; Ly, Q.S. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res. 2008, 42, 1511–1522. [Google Scholar] [CrossRef]
- Kang, J.H.; Yoon, T.-U.; Kim, S.-Y.; Kim, M.-B.; Kim, H.-J.; Yang, H.-C.; Bae, Y.-S. Extraordinarily selective adsorption of CO2 over N2 in a polyethyleneimine-impregnated NU-1000 material. Microporous Mesoporous Mater. 2019, 281, 84–91. [Google Scholar] [CrossRef]
- Yee, K.K.; Reimer, N.; Liu, J.; Cheng, S.Y.; Yiu, S.M.; Weber, J.; Stock, N.; Xu, Z. Effective mercury sorption by thiol-laced metal-organic frameworks: In strong acid and the vapor phase. J. Am. Chem. Soc. 2013, 135, 7795–7798. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, M.; Rout, K.; Mohapatra, B.K.; Anand, S. Sorption behavior of Pb(II) and Cd(II) on iron ore slime and characterization of metal ion loaded sorbent. J. Hazard. Mater. 2009, 166, 1506–1513. [Google Scholar] [CrossRef]
- Mahmud, H.N.M.E.; Huq, A.K.O.; Yahya, R.B. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: A review. RSC Adv. 2016, 6, 14778–14791. [Google Scholar] [CrossRef]
- Wang, N.; Xu, X.; Li, H.; Zhai, J.; Yuan, L.; Zhang, K.; Yu, H. Preparation and application of a xanthate-modified thiourea chitosan sponge for the removal of Pb(II) from aqueous solutions. Ind. Eng. Chem. Res. 2016, 55, 4960–4968. [Google Scholar] [CrossRef]
- Errahali, M.; Gatti, G.; Tei, L.; Paul, G.; Rolla, G.A.; Canti, L.; Fraccarollo, A.; Cossi, M.; Comotti, A.; Sozzani, P.; et al. Microporous hyper-cross-linked aromatic polymers designed for methane and carbon dioxide adsorption. J. Phys. Chem. C 2014, 118, 28699–28710. [Google Scholar] [CrossRef]
- Shi, Y.-Q.; Zhu, J.; Liu, X.-Q.; Geng, J.-C.; Sun, L.-B. Molecular template-directed synthesis of microporous polymer networks for highly selective CO2 capture. ACS Appl. Mater. Interfaces 2014, 6, 20340–20349. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-B.; Kang, Y.-H.; Shi, Y.-Q.; Jiang, Y.; Liu, X.-Q. Highly selective capture of the greenhouse gas CO2 in polymers. ACS Sustain. Chem. Eng. 2015, 3, 3077–3085. [Google Scholar] [CrossRef]
- Schwab, M.G.; Lennert, A.; Pahnke, J.; Jonschker, G.; Koch, M.; Senkovska, I.; Rehahn, M.; Kaskel, S. Nanoporous copolymer networks through multiple Friedel-Crafts-alkylation-studies on hydrogen and methane storage. J. Mater. Chem. 2011, 21, 2131–2135. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Lee, Y.-R.; Ahn, W.-S. Microporous amine-functionalized aromatic polymers and their carbonized products for CO2 adsorption. Chem. Eng. J. 2017, 319, 65–74. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Zhao, G.; Ren, X.; Gao, X.; Tan, X.; Li, J.; Chen, C.; Huang, Y.; Wang, X. Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans. 2011, 40, 10945–10952. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Li, S.; Yang, Z.; Zhang, X.; Zhuang, Y.; Zhu, Q.; Cai, D.; Qin, P.; Baeyens, J. Triazine-based N-rich porous covalent organic polymer for the effective detection and removal of Hg (II) from an aqueous solution. Chem. Eng. J. 2021, 426, 130757. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, X.; Yao, X.; Ji, H. Thiourea modified hyper-crosslinked polystyrene resin for heavy metal ions removal from aqueous solutions. J. Appl. Polym. Sci. 2018, 135, 45568. [Google Scholar] [CrossRef]
- He, Y.; Liu, Q.; Hu, J.; Zhao, C.; Peng, C.; Yang, Q.; Wang, H.; Liu, H. Efficient removal of Pb(II) by amine functionalized porous organic polymer through post-synthetic modification. Sep. Purif. Technol. 2017, 180, 142–148. [Google Scholar] [CrossRef]
- Alguacil, F.; Alcaraz, L.; García-Díaz, I.; López, F. Removal of Pb2+ in wastewater via adsorption onto an activated carbon produced from winemaking waste. Metals 2018, 8, 697. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Ding, L.; Luo, J. Adsorptive removal of Pb(II) ions from aqueous samples with amino-functionalization of Metal–Organic Frameworks MIL-101(Cr). J. Chem. Eng. Data 2015, 60, 1732–1743. [Google Scholar] [CrossRef]
- Chen, M.; Nong, S.; Zhao, Y.; Riaz, M.S.; Xiao, Y.; Molokeev, M.S.; Huang, F. Renewable P-type zeolite for superior absorption of heavy metals: Isotherms, kinetics, and mechanism. Sci. Total Environ. 2020, 726, 138535. [Google Scholar] [CrossRef]
- Guo, H.; Ren, Y.; Sun, X.; Xu, Y.; Li, X.; Zhang, T.; Kang, J.; Liu, D. Removal of Pb2+ from aqueous solutions by a high-efficiency resin. Appl. Surf. Sci. 2013, 283, 660–667. [Google Scholar] [CrossRef]
- Goswami, M.; Borah, L.; Mahanta, D.; Phukan, P. Equilibrium modeling, kinetic and thermodynamic studies on the adsorption of Cr(VI) using activated carbon derived from matured tea leaves. J. Porous Mater. 2014, 21, 1025–1034. [Google Scholar] [CrossRef]
- Aguila, B.; Sun, Q.; Perman, J.A.; Earl, L.D.; Abney, C.W.; Elzein, R.; Schlaf, R.; Ma, S. Efficient mercury capture using functionalized porous organic polymer. Adv. Mater. 2017, 29, 1700665. [Google Scholar] [CrossRef]
- Masoumi, A.; Hemmati, K.; Ghaemy, M. Structural modification of acrylonitrile–butadiene–styrene waste as an efficient nanoadsorbent for removal of metal ions from water: Isotherm, kinetic and thermodynamic study. RSC Adv. 2015, 5, 1735–1744. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. J. Chem. Eng. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Gu, S.; Wang, L.; Mao, X.; Yang, L.; Wang, C. Selective Adsorption of Pb(II) from Aqueous Solution by Triethylenetetramine-Grafted Polyacrylamide/Vermiculite. Materials 2018, 11, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.; Li, W.; Deng, D.; Chen, W.; Li, H.; Wei, C.; Tang, Y. Efficient removal of lead from highly acidic wastewater by periodic ion imprinted mesoporous SBA-15 organosilica combining metal coordination and co-condensation. J. Mater. Chem. A 2015, 3, 9789–9798. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, R.; Sun, Y.; Zhou Baig, S.A.; Xu, X. Multifunctional nanocomposites Fe3O4@SiO2-EDTA for Pb(II) and Cu(II) removal from aqueous solutions. Appl. Surf. Sci. 2016, 369, 267–276. [Google Scholar] [CrossRef]
- Xiong, C.; Yao, C. Synthesis, characterization and application of triethylenetetramine modified polystyrene resin in removal of mercury, cadmium and lead from aqueous solutions. J. Chem. Eng. 2009, 155, 844–850. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Wang, M.; Ge, X. Fabrication of fibrous amidoxime-functionalized mesoporous silica microsphere and its selectively adsorption property for Pb(2+) in aqueous solution. J. Hazard. Mater. 2015, 297, 66–73. [Google Scholar] [CrossRef]
- Kumar, N.; Fosso-Kankeu, E.; Ray, S.S. Achieving controllable MoS2 nanostructures with increased interlayer spacing for efficient removal of Pb(II) from aquatic systems. ACS Appl. Mater. Interfaces 2019, 11, 19141–19155. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Z.; Xiao, B.; Gu, P.; Yao, W.; Xing, J.; Asiri, A.M.; Alamry, K.A.; Wang, X.; Wang, S. Enhanced removal of lead ions from aqueous solution by iron oxide nanomaterials with cobalt and nickel doping. J. Clean. Prod. 2019, 211, 1250–1258. [Google Scholar] [CrossRef]
- Ren, H.; Li, B.; Neckenig, M.; Wu, D.; Li, Y.; Ma, Y.; Li, X.; Zhang, N. Efficient lead ion removal from water by a novel chitosan gel-based sorbent modified with glutamic acid ionic liquid. Carbohydr. Polym. 2019, 207, 737–746. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Mudhoo, A.; Ali Khan, M.; Otero, M.; Bundhoo, Z.M.A.; Patel, M.; Srivastava, A.; Navarathna, C.; Mlsna, T.; Mohan, D.; et al. Smart adsorbents for aquatic environmental remediation. Small 2021, 17, 1–31. [Google Scholar] [CrossRef] [PubMed]








| Sample | Yield (%, wt.) | SBET (m2/g) | Vtot (cm3/g) | EA (%, wt.) | ||
|---|---|---|---|---|---|---|
| N | C | H | ||||
| NUT-21 | 87.3 | 722 | 1.37 | 0 | 85.45 | 4.55 |
| NUT-21-TETA | 79.8 | 665 | 1.05 | 9.06 | 69.36 | 6.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Xia, J.; He, J.; Qi, K.; Peng, A.; Liu, Y. Highly Efficient Capture of Heavy Metal Ions on Amine-Functionalized Porous Polymer Gels. Gels 2023, 9, 297. https://doi.org/10.3390/gels9040297
He X, Xia J, He J, Qi K, Peng A, Liu Y. Highly Efficient Capture of Heavy Metal Ions on Amine-Functionalized Porous Polymer Gels. Gels. 2023; 9(4):297. https://doi.org/10.3390/gels9040297
Chicago/Turabian StyleHe, Xue, Jumu Xia, Jieli He, Kezhen Qi, Anzhong Peng, and Yong Liu. 2023. "Highly Efficient Capture of Heavy Metal Ions on Amine-Functionalized Porous Polymer Gels" Gels 9, no. 4: 297. https://doi.org/10.3390/gels9040297