Eco-Friendly Starch Composite Supramolecular Alginate–Ca2+ Hydrogel as Controlled-Release P Fertilizer with Low Responsiveness to Multiple Environmental Stimuli
Abstract
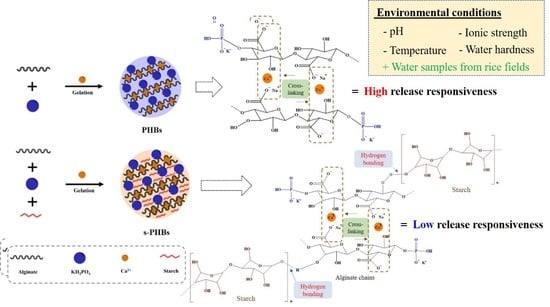
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of s-PHBs
2.3. Study of the Stability
2.4. Study of Phosphate Release
2.5. Characterization
3. Results and Discussion
3.1. Optimization for Preparing PHBs and s-PHBs
3.2. Surface Morphology of PHBs and s-PHBs
3.3. Chemical Structure of PHBs and s-PHBs
3.4. Stability of PHBs and s-PHBs
3.5. Release Behavior of PHBs and s-PHBs
3.6. Release Responsiveness of PHBs and s-PHBs to Environmental Conditions
3.6.1. Effect of pH
3.6.2. Effect of Temperature
3.6.3. Effect of Ionic Strength
3.6.4. Effect of Water Hardness
3.7. Applicability of s-PHBs in Agricultural Water Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights; United Nations: New York, NY, USA, 2019; p. 2. Available online: https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf (accessed on 14 February 2023).
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, X. Impact of fertilizer on rice yield and soil fertility in China. Sustainability 2018, 10, 1114. [Google Scholar]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Bergek, J.; Trojer, M.A.; Uhr, H.; Nordstierna, L. Controlled release of a microencapsulated arduous semi-hydrophobic active from coatings: Superhydrophilic polyelectrolyte shells as globally rate-determining barriers. J. Control. Release 2016, 225, 31–39. [Google Scholar] [CrossRef]
- Everaert, M.; Warrinnier, R.; Baken, S.; Gustafsson, J.P.; De Vos, D.; Smolders, E. Phosphate-exchanged Mg-Al layered double hydroxides: A new slow release phosphate fertilizer. ACS Sustain. Chem. Eng. 2016, 4, 4280–4287. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Guimarães, G.G.F.; Majaron, V.F.; Ribeiro, C. Controlled release of phosphate from layered double hydroxide structures: Dynamics in soil and application as smart fertilizer. ACS Sustain. Chem. Eng. 2018, 6, 5152–5161. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Cheng, D.; Gao, B.; Wan, Y.; Li, C.Y.; Yao, Y.; Xie, J.; Liu, L. Multifunctional slow-release fertilizer prepared from lignite activated by a 3D-molybdate-sulfur hierarchical hollow nanosphere catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10533–10543. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Constantino, V.R.L.; Pinto, F.G.; Vergütz, L.; Tronto, J.; da Costa, L.M. Layered double hydroxides: New technology in phosphate fertilizers based on nanostructured materials. ACS Sustain. Chem. Eng. 2017, 5, 399–409. [Google Scholar] [CrossRef]
- Anstoetz, M.; Rose, T.J.; Clark, M.W.; Yee, L.H.; Raymond, C.A.; Vancov, T. Novel applications for oxalate-phosphate-amine metalorganic-frameworks (OPA-MOFs): Can an iron-based OPA-MOF be used as slow-release fertilizer? PLoS ONE 2015, 10, e0144169. [Google Scholar] [CrossRef]
- Da Cruz, D.F.; Bortoletto-Santos, R.; Guimaraes, G.G.F.; Polito, W.L.; Ribeiro, C. Role of polymeric coating on the phosphate availability as a fertilizer: Insight from phosphate release by Castor polyurethane coatings. J. Agric. Food Chem. 2017, 65, 5890–5895. [Google Scholar] [CrossRef]
- Tao, S.; Liu, J.; Jin, K.; Qiu, X.; Zhang, Y.; Ren, X.; Hu, S. Preparation and characterization of triple polymer-coated controlled-release urea with water-retention property and enhanced durability. J. Appl. Polym. Sci. 2011, 120, 2103–2111. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Jarosiewicz, A. Use of polysulfone in controlled-release NPK fertilizer formulations. J. Agric. Food Chem. 2002, 50, 4634–4639. [Google Scholar] [CrossRef] [PubMed]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–95. [Google Scholar]
- Guimaraes, G.G.F.; Klaic, R.; Giroto, A.S.; Majaron, V.F.; Avansi, W.; Farinas, C.S.; Ribeiro, C. Smart fertilization based on sulfur-phosphate composites: Synergy among materials in a structure with multiple fertilization roles. ACS Sustain. Chem. Eng. 2018, 6, 12187–12196. [Google Scholar] [CrossRef]
- Yukhajon, P.; Somboon, T.; Sansuk, S. Fabrication of porous phosphate/carbonate composites: Smart fertilizer with bimodal controlled-release kinetics and glyphosate adsorption ability. ACS Omega 2022, 7, 15625–15636. [Google Scholar] [CrossRef]
- Lin, W.; Kluzek, M.; Luster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef]
- Feng, C.; Lü, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Gao, N.; Liu, M.; Wu, L. “Smart” fertilizer with temperature- and pH-responsive behavior via surface-initiated polymerization for controlled release of nutrients. ACS Sustain. Chem. Eng. 2015, 3, 3157–3166. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chen, C.; Liu, B.; Cai, D.; Wu, Z. Fabrication of a pH-responsively controlled-release pesticide using an attapulgite-based hydrogel. ACS Sustain. Chem. Eng. 2018, 6, 1192–1201. [Google Scholar] [CrossRef]
- Ye, Z.; Guo, J.; Wu, D.; Tan, M.; Xiong, X.; Yin, Y.; He, G. Photoresponsive shell cross-linked micelles based on carboxymethyl chitosan and their application in controlled release of pesticide. Carbohydr. Polym. 2015, 132, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, G.; Dai, Z.; Xiang, Y.; Liu, B.; Bian, P.; Zheng, K.; Wu, Z.; Cai, D. Fabrication of light-responsively controlled-release herbicide using a Nanocomposite. Chem. Eng. J. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilisers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, B.R.; Bacalhau, F.B.; dos Santos Pereira, T.; Souza, C.F.; Faez, R. Chitosan-montmorillonite microspheres: A sustainable fertilizer delivery system. Carbohydr. Polym. 2015, 127, 340–346. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Córdoba, A.L.; Deladino, L.; Martino, M. Effect of starch filler on calcium alginate hydrogels loaded with yerba mate antioxidants. Carbohydr. Polym. 2013, 95, 315–323. [Google Scholar] [CrossRef]
- Lü, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Gao, N.; Feng, C.; Wei, Y.; Wu, L.; Liu, M. Synthesis of a starch derivative and its application in fertilizer for slow nutrient release and water-holding. RSC Adv. 2014, 4, 51208–51214. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Lin, Z.T.; Zheng, X.L.; Jiang, G.B.; Fang, Y.S.; Mao, X.Y.; Liao, Z.W. Starch derivative-based superabsorbent with integration of water-retaining and controlled-release fertilizers. Carbohydr. Polym. 2003, 92, 1367–1376. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Costa, M.M.; Cabral-Albuquerque, E.C.; Alves, T.L.; Pinto, J.C.; Fialho, R.L. Use of polyhydroxybutyrate and ethyl cellulose for coating of urea granules. J. Agric. Food Chem. 2013, 61, 9984–9991. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Rojas, O.J.; Pihlajaniemi, V.; Lintinen, K.; Österberg, M. Calcium chelation of lignin from pulping spent liquor for water-resistant slow-release urea fertilizer systems. ACS Sustain. Chem. Eng. 2017, 5, 1054–1061. [Google Scholar] [CrossRef]
- González, M.; Cea, M.; Medina, J.; González, A.; Diez, M.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Sci. Total Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, X.; Yang, X.; Liu, H.; Yao, J. An eco-friendly slow-release urea fertilizer based on waste mulberry branches for potential agriculture and horticultureapplications. ACS Sustain. Chem. Eng. 2014, 2, 1871–1878. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ni, B.; Zhang, X.; Wang, Y. Slow-release nitrogen and boron fertilizer from a functional superabsorbent formulation based on wheat straw and attapulgite. Chem. Eng. J. 2011, 167, 342–348. [Google Scholar] [CrossRef]
- Jia, X.; Ma, Z.Y.; Zhang, G.X.; Hu, J.M.; Liu, Z.Y.; Wang, H.Y.; Zhou, F. Polydopamine film coated controlled-release multielement compound fertilizer based on musselinspired chemistry. J. Agric. Food Chem. 2013, 61, 2919–2924. [Google Scholar] [CrossRef]
- Perez, J.J.; Francois, N.J. Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohydr. Polym. 2016, 148, 134–142. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Ni, B.; Xie, L. κ-Carrageenan–sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind. Eng. Chem. Res. 2012, 51, 1413–1422. [Google Scholar] [CrossRef]
- Riyajan, S.A.; Sasithornsonti, Y.; Phinyocheep, P. Green natural rubber-g-modified starch for controlling urea release. Carbohydr. Polym. 2012, 89, 251–258. [Google Scholar] [CrossRef]
- Gissawong, N.; Sansuk, S.; Srijaranai, S. The Aalternative use of layered double hydroxides as extraction medium coupled with microcomplexation for determination of phosphate in water samples. Spectrochim. Acta Part A 2017, 173, 994–1000. [Google Scholar] [CrossRef]
- Xiang, Y.; Han, J.; Zhang, G.; Zhan, F.; Cai, D.; Wu, Z. Efficient synthesis of starch-regulated porous calcium carbonate microspheres as a carrier for slow-release herbicide. ACS Sustain. Chem. Eng. 2018, 6, 3649–3658. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, F. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Ahmed, S.; Arshad, T.; Zada, A.; Afzal, A.; Khan, M.; Hussain, A.; Hassan, M.; Ali, M.; Xu, S. Preparation and characterization of a novel sulfonated titanium oxide incorporated chitosan nanocomposite membranes for fuel cell application. Membranes 2021, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Draget, K.I.; Skjåk-Bræk, G.; Smidsrød, O. Alginate based new materials. Int. J. Biol. Macromol. 1997, 21, 47–55. [Google Scholar] [CrossRef]
- Jančaitienė, K.; Šlinkšienė, R. KH2PO4 crystallisation from potassium chloride and ammonium dihydrogen phosphate. Pol. J. Chem. Technol. 2016, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, X.; Zhou, Y.; Hai, C.; Shen, Y.; Ren, X.; Zeng, J. Calcium chloride hexahydrate/diatomite/paraffin as composite shape-stabilized phasechange material for thermal energy storage. Energy Fuels 2018, 32, 916–921. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Fagbohun, I.K.; Muftaudeen, T.K.; Swaray, S.; Haliru, B.S. Superabsorbent Polymer Hydrogels for Sustainable Agriculture: A Review. Horticulturae 2022, 8, 605. [Google Scholar] [CrossRef]
- Boontheekul, T.; Kong, H.J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef]
- Dos Santos Araújo, P.; Belini, G.B.; Mambrini, G.P.; Yamaji, F.M.; Waldman, W.R. Thermal degradation of calcium and sodium alginate: A greener synthesis towards calcium oxide micro/nanoparticles. Int. J. Biol. Macromol. 2019, 140, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Dollimore, D.; Heon, K. Comparative thermal analysis study of two biopolymers, starch and cellulose. J. Therm. Anal. 1997, 50, 7–17. [Google Scholar] [CrossRef]
- Blennow, A.; Nielsen, T.H.; Baunsgaard, L.; Mikkelsen, R.; Engelsen, S.B. Starch phosphorylation: A new front line in starch research. Trends Plant Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef] [PubMed]










| Model Parameter | First-Order | Higuchi | Ritger–Peppas | Parabolic Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | R2 | k | R2 | k | R2 | n | k | R2 | b | |
| KH2PO4 | 0.0078 | 0.9852 | 2.8615 | 0.7868 | 95.9867 | 0.7001 | 0.0222 | 163.1695 | 0.9607 | −56.0595 |
| 18-46-0 fertilizer | 0.0087 | 0.9844 | 3.2899 | 0.8895 | 94.0052 | 0.9122 | 0.0283 | 157.5069 | 0.9642 | −53.3066 |
| PHBs-DI | 0.0097 | 0.7297 | 3.5643 | 0.9199 | 36.4738 | 0.9741 | 0.1153 | 48.0215 | 0.9801 | −9.2635 |
| PHBs-pH = 5 | 0.0155 | 0.5923 | 4.7828 | 0.8612 | 25.9484 | 0.9283 | 0.2012 | 29.5638 | 0.9974 | −4.1631 |
| s-PHBs-DI | 0.0117 | 0.6828 | 5.0542 | 0.8950 | 40.4531 | 0.9535 | 0.1432 | 51.7961 | 0.9721 | −9.4696 |
| s-PHBs-pH = 5 | 0.0170 | 0.5727 | 3.7395 | 0.8620 | 18.1305 | 0.8955 | 0.2193 | 19.7474 | 0.9889 | −2.3884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiamwong, S.; Yukhajon, P.; Noisong, P.; Subsadsana, M.; Sansuk, S. Eco-Friendly Starch Composite Supramolecular Alginate–Ca2+ Hydrogel as Controlled-Release P Fertilizer with Low Responsiveness to Multiple Environmental Stimuli. Gels 2023, 9, 204. https://doi.org/10.3390/gels9030204
Tiamwong S, Yukhajon P, Noisong P, Subsadsana M, Sansuk S. Eco-Friendly Starch Composite Supramolecular Alginate–Ca2+ Hydrogel as Controlled-Release P Fertilizer with Low Responsiveness to Multiple Environmental Stimuli. Gels. 2023; 9(3):204. https://doi.org/10.3390/gels9030204
Chicago/Turabian StyleTiamwong, Supattra, Pratchayaporn Yukhajon, Pittayagorn Noisong, Maliwan Subsadsana, and Sira Sansuk. 2023. "Eco-Friendly Starch Composite Supramolecular Alginate–Ca2+ Hydrogel as Controlled-Release P Fertilizer with Low Responsiveness to Multiple Environmental Stimuli" Gels 9, no. 3: 204. https://doi.org/10.3390/gels9030204