Development of Efficient Sodium Alginate/Polysuccinimide-Based Hydrogels as Biodegradable Acetaminophen Delivery Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Alg-PSI
2.2. Rheological Properties of Hydrogels
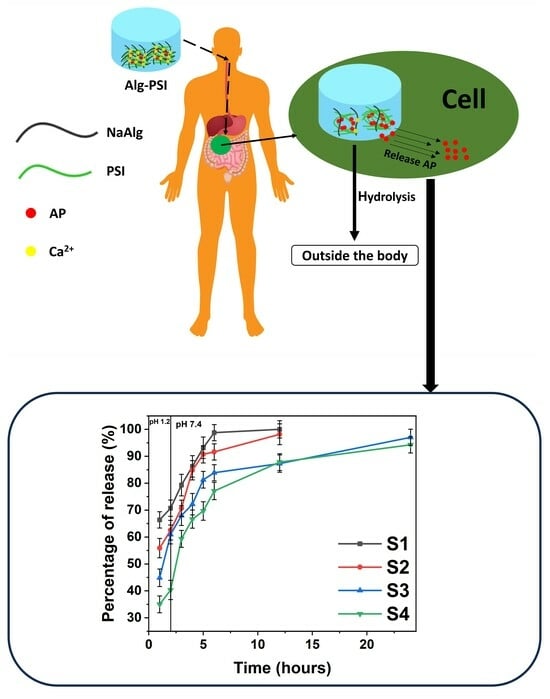
2.3. Drug Loading Efficiency and Degradation Tests to Assess Drug Release
2.4. Cell Viability of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Alginate (Alg) Hydrogels
4.2.1. Synthesis of Alg
4.2.2. Synthesis of Acetaminophen-Loaded Alg (AP/Alg) Hydrogels
4.3. Synthesis of Alg-PSI Hydrogels
4.3.1. Synthesis of PSI
4.3.2. Synthesis of Complex Alg-PSI Hydrogels
4.3.3. Synthesis of AP-Loaded Complex Alg-PSI (AP/Alg-PSI) Hydrogels
4.4. Characterization
4.5. Rheological Tests of Hydrogels
4.6. Degradation Analyses of Hydrogels
4.7. AP Encapsulation Efficiency
4.8. Acetaminophen Release Tests
4.9. In Vitro Cytotoxicity
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, G.; Huang, L.; Zhang, Z. The Molecular Mechanisms of Acetaminophen-Induced Hepatotoxicity and Its Potential Therapeutic Targets. Exp. Biol. Med. 2023, 248, 412–424. [Google Scholar] [CrossRef]
- Adhikary, S.; Al Hoque, A.; Ray, M.; Paul, S.; Hossain, A.; Goswami, S.; Dey, R. Investigation of Paracetamol Entrapped Nanoporous Silica Nanoparticles in Transdermal Drug Delivery System. Appl. Biochem. Biotechnol. 2023, 195, 4712–4727. [Google Scholar] [CrossRef]
- Chen, C.; Wu, H.; Li, Q.; Liu, M.; Yin, F.; Wu, M.; Wei, X.; Wang, H.; Zha, Z.; Wang, F. Manganese Prussian Blue Nanozymes with Antioxidant Capacity Prevent Acetaminophen-Induced Acute Liver Injury. Biomater. Sci. 2023, 11, 2348–2358. [Google Scholar] [CrossRef]
- Jaeschke, H.; Adelusi, O.B.; Akakpo, J.Y.; Nguyen, N.T.; Sanchez-Guerrero, G.; Umbaugh, D.S.; Ding, W.X.; Ramachandran, A. Recommendations for the Use of the Acetaminophen Hepatotoxicity Model for Mechanistic Studies and How to Avoid Common Pitfalls. Acta Pharm. Sin. B 2021, 11, 3740–3755. [Google Scholar] [CrossRef]
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute Liver Failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef]
- Kulsoom, R.; Sarfraz, M.; Afzal, A.; Farooq, M.; Adnan, S.; Ashraf, M.U.; Khan, S.A. Synthesis of Calcium Carbonate-Quince Bio-Composite for Programmed and on-Demand Drug Release of Paracetamol at Target Site: A Green Chemistry Approach. Polym. Bull. 2023, 80, 6965–6988. [Google Scholar] [CrossRef]
- Rainsford, K.D.; Whitehouse, M.W. Paracetamol [Acetaminophen]-Induced Gastrotoxicity: Revealed by Induced Hyperacidity in Combination with Acute or Chronic Inflammation. Inflammopharmacology 2006, 14, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Shimono, N.; Takatori, T.; Ueda, M.; Mori, M.; Higashi, Y.; Nakamura, Y. Chitosan Dispersed System for Colon-Specific Drug Delivery. Int. J. Pharm. 2002, 245, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.A.; Duy Le, T.M.; Ho, H.G.V.; To, T.C.T.; Nguyen, V.V.L.; Huynh, D.P.; Lee, D.S. A Novel Injectable pH-Temperature Sensitive Hydrogel Containing Chitosan-Insulin Electrosprayed Nanosphere Composite for an Insulin Delivery System in Type i Diabetes Treatment. Biomater. Sci. 2020, 8, 3830–3843. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, S.; Li, S.; Pan, H. Genipin-Cross-Linked Hydrogels Based on Biomaterials for Drug Delivery: A Review. Biomater. Sci. 2021, 9, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Levkovskyi, I.O.; Mochizuki, S.; Zheng, A.; Zhang, X.; Zhang, F. Lipoic Acid-Based Poly(Disulfide)s: Synthesis and Biomedical Applications. Nano TransMed 2023, 2, 100006. [Google Scholar] [CrossRef]
- Jing, S.; Liu, Y.; Ye, Z.; Ghaleb Al-bashari, A.A.; Zhou, H.; He, Y. Ferrostatin-1 Loaded Gelatin Methacrylate Scaffold Promotes Recovery from Spinal Cord Injury via Inhibiting Apoptosis and Ferroptosis. Nano TransMed 2023, 2, 100005. [Google Scholar] [CrossRef]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Shaw, J.E.; Arno, M.C.; Hoyland, J.A.; Richardson, S.M.; Dove, A.P. Robust Alginate/Hyaluronic Acid Thiol-Yne Click-Hydrogel Scaffolds with Superior Mechanical Performance and Stability for Load-Bearing Soft Tissue Engineering. Biomater. Sci. 2020, 8, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Uthappa, U.T.; Altalhi, T.; Jung, H.Y.; Han, S.S.; Kurkuri, M.D. Alginate Based Polymeric Systems for Drug Delivery, Antibacterial/Microbial, and Wound Dressing Applications. Mater. Today Commun. 2022, 33, 104813. [Google Scholar] [CrossRef]
- Mahmood, A.; Mahmood, A.; Ibrahim, M.A.; Hussain, Z.; Ashraf, M.U.; Salem-Bekhit, M.M.; Elbagory, I. Development and Evaluation of Sodium Alginate/Carbopol 934P-Co-Poly (Methacrylate) Hydrogels for Localized Drug Delivery. Polymers 2023, 15, 311. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P. In Vitro Drug Release Profiles of PH-Sensitive Hydroxyethylacryl Chitosan/Sodium Alginate Hydrogels Using Paracetamol as a Soluble Model Drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef]
- Pervez, S.; Nawaz, M.A.; Aman, A.; Qayyum, S.; Nawaz, F.; Qader, S.A.U. Agarose Hydrogel Beads: An Effective Approach to Improve the Catalytic Activity, Stability and Reusability of Fungal Amyloglucosidase of GH15 Family. Catal. Lett. 2018, 148, 2643–2653. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic Hydrocolloids for the Intestinal Delivery of Protein Drugs: Alginate and Chitosan—A Review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Rubio, M.R.; Ghaly, E.S. In-Vitro Release of Acetaminophen from Sodium Alginate Controlled Release Pellets. Drug Dev. Ind. Pharm. 1994, 20, 1239–1251. [Google Scholar] [CrossRef]
- Sharma, S.; Sarkar, G.; Srestha, B.; Chattopadhyay, D.; Bhowmik, M. In-Situ Fast Gelling Formulation for Oral Sustained Drug Delivery of Paracetamol to Dysphagic Patients. Int. J. Biol. Macromol. 2019, 134, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Nayak, A.K. Alginate-Inorganic Composite Particles as Sustained Drug Delivery Matrices. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 39–74. ISBN 9780128137581. [Google Scholar]
- Jain, S.; Datta, M. Montmorillonite-Alginate Microspheres as a Delivery Vehicle for Oral Extended Release of Venlafaxine Hydrochloride. J. Drug Deliv. Sci. Technol. 2016, 33, 149–156. [Google Scholar] [CrossRef]
- Iliescu, R.I.; Andronescu, E.; Ghitulica, C.D.; Voicu, G.; Ficai, A.; Hoteteu, M. Montmorillonite-Alginate Nanocomposite as a Drug Delivery System—Incorporation and in Vitro Release of Irinotecan. Int. J. Pharm. 2014, 463, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Wang, C.Q.; Zhuo, R.X.; Cheng, S.X. Multi-Drug Delivery System Based on Alginate/Calcium Carbonate Hybrid Nanoparticles for Combination Chemotherapy. Colloids Surf. B Biointerfaces 2014, 123, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Currie, S.; Kim, S.; Gu, X.; Ren, X.; Lin, F.; Liu, S.; Yang, C.; Kim, J.H.; Liu, S. Mucus-Penetrating PEGylated Polysuccinimide-Based Nanocarrier for Intravaginal Delivery of SiRNA Battling Sexually Transmitted Infections. Colloids Surf. B Biointerfaces 2020, 196, 111287. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.R.; MacKrell, E.J.; Forsthoefel, C.P.; Jensen, S.P.; Chen, M.; Moore, G.A.; He, Z.L.; Sumerlin, B.S. Biodegradable and PH-Responsive Nanoparticles Designed for Site-Specific Delivery in Agriculture. Biomacromolecules 2015, 16, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hu, P.; Xu, Y.; Xiao, C.; Chen, Z.; Liu, X.; Jia, J.; Xu, H. Phloem Delivery of Fludioxonil by Plant Amino Acid Transporter-Mediated Polysuccinimide Nanocarriers for Controlling Fusarium Wilt in Banana. J. Agric. Food Chem. 2021, 69, 2668–2678. [Google Scholar] [CrossRef]
- Barczikai, D.; Kacsari, V.; Domokos, J.; Szabó, D.; Jedlovszky-Hajdu, A. Interaction of Silver Nanoparticle and Commonly Used Anti-Inflammatory Drug within a Poly(Amino Acid) Derivative Fibrous Mesh. J. Mol. Liq. 2021, 322, 114575. [Google Scholar] [CrossRef]
- Adelnia, H.; Blakey, I.; Little, P.J.; Ta, H.T. Poly(Succinimide) Nanoparticles as Reservoirs for Spontaneous and Sustained Synthesis of Poly(Aspartic Acid) under Physiological Conditions: Potential for Vascular Calcification Therapy and Oral Drug Delivery. J. Mater. Chem. B 2023, 11, 2650–2662. [Google Scholar] [CrossRef]
- Heading, R.C.; Nimmo, J.; Prescott, L.F.; Tothill, P. The Dependence of Paracetamol Absorption on the Rate of Gastric Emptying. Br. J. Pharmacol. 1973, 47, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Lim, C.W.; Kim, D. Bone Targeting Nano-Aggregates Prepared from Self-Assembled Polyaspartamide Graft Copolymers for PH Sensitive DOX Delivery. Biomater. Sci. 2021, 9, 1660–1667. [Google Scholar] [CrossRef]
- Kim, M.; Shin, S.W.; Lim, C.W.; Kim, J.; Um, S.H.; Kim, D. Polyaspartamide-Based Graft Copolymers Encapsulating Iron Oxide Nanoparticles for Imaging and Fluorescence Labelling of Immune Cells. Biomater. Sci. 2017, 5, 305–312. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Shavandi, A. Polysuccinimide and Its Derivatives: Degradable and Water Soluble Polymers (Review). Eur. Polym. J. 2018, 109, 43–54. [Google Scholar] [CrossRef]
- Almurisi, S.H.; Doolaanea, A.A.; Akkawi, M.E.; Chatterjee, B.; Sarker, M.Z.I. Taste Masking of Paracetamol Encapsulated in Chitosan-Coated Alginate Beads. J. Drug Deliv. Sci. Technol. 2020, 56, 101520. [Google Scholar] [CrossRef]
- Tóth, K.; Fekete, N.; Klaudia Simon, V.; Tóth, B.; Ferenc Kovács, Á.; Pállinger, É.; Antal, I.; Kőhidai, L.; Jedlovszky-Hajdú, A.; Juriga, D.; et al. Different Implantable Electrospun Meshes for Simultaneous Application of Prednisone and Doxorubicin. J. Mol. Liq. 2023, 381, 121854. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. PH-Sensitive Sodium Alginate/Poly(Vinyl Alcohol) Hydrogel Beads Prepared by Combined Ca2+ Crosslinking and Freeze-Thawing Cycles for Controlled Release of Diclofenac Sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef]
- Pasparakis, G.; Bouropoulos, N. Swelling Studies and in Vitro Release of Verapamil from Calcium Alginate and Calcium Alginate-Chitosan Beads. Int. J. Pharm. 2006, 323, 34–42. [Google Scholar] [CrossRef]
- Weng, L.; Tessier, S.N.; Swei, A.; Stott, S.L.; Toner, M. Controlled Ice Nucleation Using Freeze-Dried Pseudomonas Syringae Encapsulated in Alginate Beads. Cryobiology 2017, 75, 1–6. [Google Scholar] [CrossRef]
- Popescu, I.N.; Poinescu, A.A.; Ungureanu, D.N.; Picu, A. Novel Developments in Advanced Materials Fields: Porous and Non-Porous Biomaterials Used in Regenerative Medicine and Tissue Engineering. Sci. Bull. Valahia Univ.-Mater. Mech. 2023, 19, 42–52. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; Xu, X.; Zhang, M.; Ma, L. Structure Regulation of Bentonite-Alginate Nanocomposites for Controlled Release of Imidacloprid. ACS Omega 2020, 5, 10068–10076. [Google Scholar] [CrossRef] [PubMed]
- Gijpferich, A. Mechanisms of Polymer Degradation and Erosion. In The Biomaterials: Silver Jubilee Compendium; Elsevier: Amsterdam, The Netherlands, 1996; Volume 17. [Google Scholar]
- Yoshioka, T.; Kawazoe, N.; Tateishi, T.; Chen, G. In Vitro Evaluation of Biodegradation of Poly(Lactic-Co-Glycolic Acid) Sponges. Biomaterials 2008, 29, 3438–3443. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.O.; Chiappini, C.; Ziemys, A.; Faust, A.M.; Kojic, M.; Liu, X.; Ferrari, M.; Tasciotti, E. Engineering Multi-Stage Nanovectors for Controlled Degradation Andtunable Release Kinetics. Biomaterials 2013, 34, 8469–8477. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Fong Wee, S. Protein Release from Alginate Matrices. In Advanced Drug Delivery Reviews; Elsevier: Amsterdam, The Netherlands, 1998; Volume 31. [Google Scholar]
- Bajpai, S.K.; Sharma, S. Investigation of Swelling/Degradation Behaviour of Alginate Beads Crosslinked with Ca2+ and Ba2+ Ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Marković, D.; Zarubica, A.; Stojković, N.; Vasić, M.; Cakić, M.; Nikolić, G. Alginates and similar exopolysaccharides in biomedical application and pharmacy: Controled delivery of drugs. Adv. Technol. 2016, 5, 39–52. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar]
- Tomida, M.; Nakato, T.; Matsunami, S.; Kakuchi, T. Convenient Synthesis of High Molecular Weight Poly(Succinimide) by Acid-Catalyses Polycondensation of L-Aspartic Acid. Polymer 1997, 18, 4733–4736. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, L.T.; Lim, S.; Lee, H.J.; Kim, I.T. Development of Efficient Sodium Alginate/Polysuccinimide-Based Hydrogels as Biodegradable Acetaminophen Delivery Systems. Gels 2023, 9, 980. https://doi.org/10.3390/gels9120980
Trinh LT, Lim S, Lee HJ, Kim IT. Development of Efficient Sodium Alginate/Polysuccinimide-Based Hydrogels as Biodegradable Acetaminophen Delivery Systems. Gels. 2023; 9(12):980. https://doi.org/10.3390/gels9120980
Chicago/Turabian StyleTrinh, Long Toan, Saebin Lim, Hyun Jong Lee, and Il Tae Kim. 2023. "Development of Efficient Sodium Alginate/Polysuccinimide-Based Hydrogels as Biodegradable Acetaminophen Delivery Systems" Gels 9, no. 12: 980. https://doi.org/10.3390/gels9120980