Activated Carbon-Incorporated Tragacanth Gum Hydrogel Biocomposite: A Promising Adsorbent for Crystal Violet Dye Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
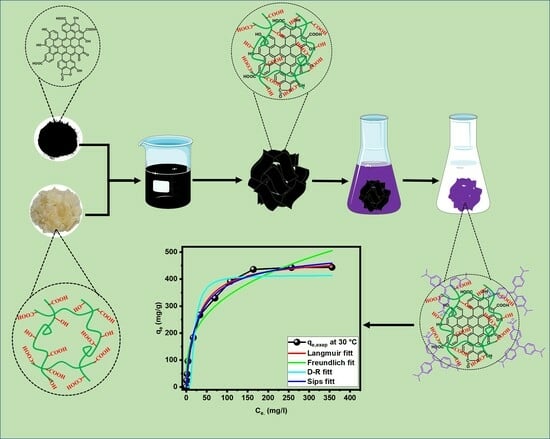
2.2. Adsorption Study
2.3. Isotherm Analysis
2.4. Kinetic Analysis
2.5. Thermodynamic Study
2.6. Adsorption Mechanism and Reusability
3. Conclusions
4. Materials and Method
4.1. Materials
4.2. Preparation Method
4.3. Adsorption Measurements
4.4. Characterization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweitzer, L.; Noblet, J. Water Contamination and Pollution. Green Chem. Incl. Approach 2018, 261–290. [Google Scholar] [CrossRef]
- Qadri, R.; Faiq, M.A.; Qadri, R.; Faiq, M.A. Freshwater Pollution: Effects on Aquatic Life and Human Health. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 15–26. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.F.; Wang, Z.; Song, L.; Yao, J. Flexible cellulose foams with a high loading of attapulgite nanorods for Cu2+ ions removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126038. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Thamer, B.M.; Aldalbahi, A.; Meera Moydeen, A.; Rahaman, M.; El-Newehy, M.H. Modified Electrospun Polymeric Nanofibers and Their Nanocomposites as Nanoadsorbents for Toxic Dye Removal from Contaminated Waters: A Review. Polymers 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Thamer, B.M.; Al-aizari, F.A.; Hameed, M.M.A. Zero-valent Ni/NiO core-shell nanosheets supported on graphene for highly efficient dye adsorption: Isotherm, kinetic and thermodynamic study. Chem. Eng. Res. Des. 2023, 197, 656–668. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater Treatment and Reuse: A Review of its Applications and Health Implications. Water Air Soil Pollut. 2021, 232, 1–28. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Lu, T.; Cao, W.; Liang, H.; Deng, Y.; Zhang, Y.; Zhu, M.; Ma, W.; Xiong, R.; Huang, C. Blow-Spun Nanofibrous Membrane for Simultaneous Treatment of Emulsified Oil/Water Mixtures, Dyes, and Bacteria. Langmuir 2022, 38, 15729–15739. [Google Scholar] [CrossRef]
- Thamer, B.M.; Al-Enizi, A.; Altaleb, H.A.; AlAnazi, N.B.; Ubaidullah, M.; El-Newehy, M.H. Hollow carbon fibers and flakes derived from Calotropis procera as adsorbents for dye removal from aqueous solutions. Mater. Chem. Phys. 2022, 279, 125752. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B.L.; Lim, L.H.; Chan, C.M. Separation of acid blue 25 from aqueous solution using water lettuce and agro-wastes by batch adsorption studies. Appl. Water Sci. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Gayathiri, M.; Pulingam, T.; Lee, K.T.; Sudesh, K. Activated carbon from biomass waste precursors: Factors affecting production and adsorption mechanism. Chemosphere 2022, 294, 133764. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Abdul, A.K.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Liang, H.; Cao, W.; Deng, Y.; Qu, Q.; Ma, W.; Xiong, R.; Huang, C. Blow-spun nanofibrous composite Self-cleaning membrane for enhanced purification of oily wastewater. J. Colloid Interface Sci. 2022, 608, 2860–2869. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; Thamer, B.M.; Rahaman, M.; El-Newehy, M.H. Self-Nitrogen-Doped Nanoporous Carbons Derived from Poly(1,5-diaminonaphthalene) for the Removal of Toxic Dye Pollutants from Wastewater: Non-Linear Isotherm and Kinetic Analysis. Polymers 2020, 12, 2563. [Google Scholar] [CrossRef]
- Khan, M.; Lo, I.M.C. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res. 2016, 106, 259–271. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Yu, F.; Yang, P.; Yang, Z.; Zhang, X.; Ma, J. Double-network hydrogel adsorbents for environmental applications. Chem. Eng. J. 2021, 426, 131900. [Google Scholar] [CrossRef]
- Thamer, B.M.; Aldalbahi, A.; Meera, M.A.; El-Newehy, M.H. In Situ Preparation of Novel Porous Nanocomposite Hydrogel as Effective Adsorbent for the Removal of Cationic Dyes from Polluted Water. Polymers 2020, 12, 3002. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Li, H.; Chen, L.; Xu, L.; Liu, J.; Geng, A.; Mei, C.; Shang, S. Graphene oxide incorporated alginate hydrogel beads for the removal of various organic dyes and bisphenol A in water. Colloid Polym. Sci. 2018, 296, 607–615. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Rodrigues, F.H.A.; Paulino, A.T.; Martins, A.F.; Fajardo, A.R. Recent advances on composite hydrogels designed for the remediation of dye-contaminated water and wastewater: A review. J. Clean. Prod. 2021, 284, 124703. [Google Scholar] [CrossRef]
- Nangia, S.; Warkar, S.; Katyal, D. A review on environmental applications of chitosan biopolymeric hydrogel based composites. J. Macromol. Sci. Part A 2018, 55, 747–763. [Google Scholar] [CrossRef]
- Polez, R.T.; Morits, M.; Jonkergouw, C.; Phiri, J.; Valle-Delgado, J.J.; Linder, M.B.; Maloney, T.; Rojas, O.J.; Österberg, M. Biological activity of multicomponent bio-hydrogels loaded with tragacanth gum. Int. J. Biol. Macromol. 2022, 215, 691–704. [Google Scholar] [CrossRef]
- Fahimirad, S. Gum tragacanth-based nanosystems for therapeutic applications. Polym. Nanosyst. Theranostic Nanosyst. 2023, 1, 367–404. [Google Scholar] [CrossRef]
- Etemadinia, T.; Barikbin, B.; Allahresani, A. Removal of congo red dye from aqueous solutions using ZnFe2O4/SiO2/Tragacanth gum magnetic nanocomposite as a novel adsorbent. Surf. Interfaces 2019, 14, 117–126. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rahimdokht, M.; Pajootan, E. Low cost hydrogels based on gum Tragacanth and TiO2 nanoparticles: Characterization and RBFNN modelling of methylene blue dye removal. Int. J. Biol. Macromol. 2019, 134, 967–975. [Google Scholar] [CrossRef]
- Sahraei, R.; Sekhavat Pour, Z.; Ghaemy, M. Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: Removal of heavy metals and dyes from water. J. Clean. Prod. 2017, 142, 2973–2984. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Hamshary, H.; Al-Deyab, S.S.; El-Newehy, M.H. Functionalized electrospun carbon nanofibers for removal of cationic dye. Arab. J. Chem. 2019, 12, 747–759. [Google Scholar] [CrossRef]
- Huang, W.; Hu, Y.; Li, Y.; Zhou, Y.; Niu, D.; Lei, Z.; Zhang, Z. Citric acid-crosslinked β-cyclodextrin for simultaneous removal of bisphenol A, methylene blue and copper: The roles of cavity and surface functional groups. J. Taiwan Inst. Chem. Eng. 2018, 82, 189–197. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Aghaie, H.; Sheykhloie, H.; Vardini, M.T.; Etemadi, H. Synthesis of CarAlg/MMt nanocomposite hydrogels and adsorption of cationic crystal violet. Carbohydr. Polym. 2013, 98, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.R.; Abu Elella, M.H.; Sabaa, M.W.; Saad, G.R. Synthesis of an efficient adsorbent hydrogel based on biodegradable polymers for removing crystal violet dye from aqueous solution. Cellulose 2018, 25, 6513–6529. [Google Scholar] [CrossRef]
- Li, S. Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly(acrylic acid-acrylamide-methacrylate) and amylose. Bioresour. Technol. 2010, 101, 2197–2202. [Google Scholar] [CrossRef]
- Qi, X.; Zeng, Q.; Tong, X.; Su, T.; Xie, L.; Yuan, K.; Xu, J.; Shen, J. Polydopamine/montmorillonite-embedded pullulan hydrogels as efficient adsorbents for removing crystal violet. J. Hazard. Mater. 2021, 402, 123359. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Synthesis of Guar gum/bentonite a novel bionanocomposite: Isotherms, kinetics and thermodynamic studies for the removal of Pb (II) and crystal violet dye. J. Mol. Liq. 2018, 249, 805–814. [Google Scholar] [CrossRef]
- Preetha, B.K.; Vishalakshi, B. Microwave assisted synthesis of karaya gum based montmorillonite nanocomposite: Characterisation, swelling and dye adsorption studies. Int. J. Biol. Macromol. 2020, 154, 739–750. [Google Scholar] [CrossRef]
- Thamer, B.M.; Abdo, H.S. Tragacanth gum-enhanced adsorption performance of polyvinyl alcohol nanofibers for cationic crystal violet dye removal. Biomass Convers. Biorefinery 2023, 1–13. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J. Mol. Liq. 2021, 323, 115034. [Google Scholar] [CrossRef]
- Aref, L.; Navarchian, A.H.; Dadkhah, D. Adsorption of Crystal Violet Dye from Aqueous Solution by Poly(Acrylamide-co-Maleic Acid)/Montmorillonite Nanocomposite. J. Polym. Environ. 2017, 25, 628–639. [Google Scholar] [CrossRef]
- Rehman, T.U.; Bibi, S.; Khan, M.; Ali, I.; Shah, L.A.; Khan, A.; Ateeq, M. Fabrication of stable superabsorbent hydrogels for successful removal of crystal violet from waste water. RSC Adv. 2019, 9, 40051–40061. [Google Scholar] [CrossRef] [PubMed]
- Loulidi, I.; Jabri, M.; Amar, A.; Kali, A.; Alrashdi, A.A.; Hadey, C.; Ouchabi, M.; Abdullah, P.S.; Lgaz, H.; Cho, Y.; et al. Comparative Study on Adsorption of Crystal Violet and Chromium (VI) by Activated Carbon Derived from Spent Coffee Grounds. Appl. Sci. 2023, 13, 985. [Google Scholar] [CrossRef]
- Thamer, B.M.; Aldalbahi, A.; Moydeen, A.M.; El-Hamshary, H.; Al-Enizi, A.M.; El-Newehy, M.H. Effective adsorption of Coomassie brilliant blue dye using poly(phenylene diamine)grafted electrospun carbon nanofibers as a novel adsorbent. Mater. Chem. Phys. 2019, 234, 133–145. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Massoudi, A.; Baghban, A.; Shokri, E. Study of adsorption of cationic dye on magnetic kappa-carrageenan/PVA nanocomposite hydrogels. J. Environ. Chem. Eng. 2014, 2, 1578–1587. [Google Scholar] [CrossRef]
- Akin, K.; Ugraskan, V.; Isik, B.; Cakar, F. Adsorptive removal of crystal violet from wastewater using sodium alginate-gelatin-montmorillonite ternary composite microbeads. Int. J. Biol. Macromol. 2022, 223, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Thamer, B.M.; Shaker, A.A.; Abdul Hameed, M.M.; Al-Enizi, A.M. Highly selective and reusable nanoadsorbent based on expansive clay-incorporated polymeric nanofibers for cationic dye adsorption in single and binary systems. J. Water Process Eng. 2023, 54, 103918. [Google Scholar] [CrossRef]
- Indana, M.K.; Gangapuram, B.R.; Dadigala, R.; Bandi, R.; Guttena, V. A novel green synthesis and characterization of silver nanoparticles using gum tragacanth and evaluation of their potential catalytic reduction activities with methylene blue and Congo red dyes. J. Anal. Sci. Technol. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Djelad, A.; Mokhtar, A.; Khelifa, A.; Bengueddach, A.; Sassi, M. Alginate-whey an effective and green adsorbent for crystal violet removal: Kinetic, thermodynamic and mechanism studies. Int. J. Biol. Macromol. 2019, 139, 944–954. [Google Scholar] [CrossRef]
- Thamer, B.M.; Al-aizari, F.A.; Abdo, H.S. Enhanced Adsorption of Textile Dyes by a Novel Sulfonated Activated Carbon Derived from Pomegranate Peel Waste: Isotherm, Kinetic and Thermodynamic Study. Molecules 2023, 28, 7712. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. Z. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Dubinin, M.; Radushkevich, L. Equation of the characteristic curve of activated charcoal. Chem. Zentr. 1947, 1, 875–890. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Wase, D.A.J.; Forster, C.F. Kinetic Studies of Competitive Heavy Metal Adsorption by Sphagnum Moss Peat. Environ. Technol. 1996, 17, 71–77. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils †. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- WJ Weber, J.M. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]








| Model | Adsorbent | |||
|---|---|---|---|---|
| 25 °C | 30 °C | 35 °C | 40 °C | |
| qe,exp (mg/g) | 427 | 444 | 450 | 406 |
| Langmuir | ||||
| qmax (mg/g) | 468.89 ± 13.25 | 482.98 ± 12.58 | 481.21 ± 12.51 | 452.19 ± 8.25 |
| KL (L/mg) | 0.033 ± 0.003 | 0.0386 ± 0.0042 | 0.0477 ± 0.0056 | 0.0304 ± 0.0022 |
| R2 | 0.9923 | 0.9930 | 0.9922 | 0.9967 |
| r-χ2 | 249.68 | 245.74 | 285.06 | 100.11 |
| Freundlich | ||||
| KF (mg/g)/(mg/L)n | 69.51 ± 13.67 | 76.22 ± 15.25 | 83.73 ± 16.98 | 61.59 ± 14.94 |
| 1/n | 3.05 ± 0.358 | 3.10 ± 0.3828 | 3.23 ± 0.4237 | 2.95 ± 0.4098 |
| R2 | 0.9571 | 0.9513 | 0.9437 | 0.9374 |
| r-χ2 | 1398.01 | 1720.84 | 2051.91 | 1880.34 |
| Dubinin–Radushkevich | ||||
| qs (mg/g) | 398.48 ± 22.48 | 414.15 ± 22.70 | 421.99 ± 21.76 | 384.06 ± 20.82 |
| KD-R (mol2/KJ2) | 69.36 ± 21.87 | 49.77 ± 14.90 | 36.01 ± 9.81 | 71.59 ± 21.94 |
| E (kJ/mol) | ||||
| R2 | 0.9278 | 0.9321 | 0.9395 | 0.9328 |
| r-χ2 | 2352.92 | 2400.43 | 2205.74 | 2017.92 |
| Sips | ||||
| qs | 455.61 ± 16.07 | 470.86 ± 15.21 | 477.37 ± 15.05 | 427.53 ± 10.81 |
| Ks | 0.0122 ± 0.0011 | 0.0140 ± 0.0099 | 0.02265 ± 0.0193 | 0.0089 ± 0.0072 |
| ns | 1.25 ± 0.2396 | 1.26 ± 0.2440 | 1.18 ± 0.2311 | 1.35 ± 0.2106 |
| R2 | 0.9956 | 0.9957 | 0.9993 | 0.9993 |
| r-χ2 | 63.66 | 59.76 | 50.87 | 44.40 |
| Adsorbent | Adsorption Conditions | qmax | Ref. |
|---|---|---|---|
| Kappa-carrageenan-sodium alginate | Co = 10–100 mg/L, pH = 6.4, T = - K | 88.8 | [33] |
| GO/polyacrylamide/sodium alginate | Co = 10–300 mg/L, pH = 8, T = 298 K | 100.3 | [34] |
| Xanthan gum/poly(N-vinyl imidazole) | Co = 450–600 mg/L, pH = 7, T = 303 K | 453 | [35] |
| Poly(acrylic acid-acrylamide-methacrylate)/amylose | Co = 1–50 mg/L, pH = 7.4, T = 298 K | 35.09 | [36] |
| Polydopamine/montmorillonite/ pullulan | Co = 50–300 mg/L, pH = -, T = - K | 112.45 | [37] |
| Guar gum/bentonite | Co = 5–50 mg/L, pH = 7.6, T = 293 K | 167.29 | [38] |
| Karaya gum /montmorillonite | Co = 20–100 mg/L, pH = 7, T = 300 K | 137.77 | [39] |
| Tragacanth gum/PVA nanofibers | Co = 25–450 mg/L, pH = 10, T = 298 K | [40] | |
| Activated carbon/tragacanth gum | Co = 25–600 mg/L, pH = 10, T = 298 K | 477.37 | This study |
| Model | Concentration | |
|---|---|---|
| 100 mg/L | 200 mg/L | |
| qe,exp (mg/g) | 90 | 179 |
| Pseudo-first-order | ||
| qe,cal (mg/g) | 77.01 ± 3.08 | 164.81 ± 3.82 |
| k1 | 0.0505 ± 0.0096 | 0.1050 ± 0.01595 |
| R2 | 0.7333 | 0.8522 |
| χ2 | 127.05 | 238.65 |
| Pseudo-second-order | ||
| qe,cal (mg/g) | 86.73 ± 2.24 | 178.42 ± 1.21 |
| K2 | 5.73 × 10−4 ± 9.71 × 10−5 | 6.29 × 10−4 ± 4.85 × 10−5 |
| R2 | 0.9998 | 0.9999 |
| r-χ2 | 7.65 | 11.26 |
| Elovich | ||
| β | 0.0959 ± 0.0037 | 0.0769 ± 0.0053 |
| α | 66.19 ± 14.51 | 21,132.93 ± 16,720.17 |
| R2 | 0.9839 | 0.9998 |
| r-χ2 | 32.67 | 25.97 |
| Temperature (K) | ∆G° (KJ/mol) | ∆H° (KJ/mol) | ∆S° (J/mol K) |
|---|---|---|---|
| 298 | −31.04 | 47.06 | 261.62 |
| 303 | −31.91 | ||
| 308 | −33.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thamer, B.M.; Al-aizari, F.A.; Abdo, H.S. Activated Carbon-Incorporated Tragacanth Gum Hydrogel Biocomposite: A Promising Adsorbent for Crystal Violet Dye Removal from Aqueous Solutions. Gels 2023, 9, 959. https://doi.org/10.3390/gels9120959
Thamer BM, Al-aizari FA, Abdo HS. Activated Carbon-Incorporated Tragacanth Gum Hydrogel Biocomposite: A Promising Adsorbent for Crystal Violet Dye Removal from Aqueous Solutions. Gels. 2023; 9(12):959. https://doi.org/10.3390/gels9120959
Chicago/Turabian StyleThamer, Badr M., Faiz A. Al-aizari, and Hany S. Abdo. 2023. "Activated Carbon-Incorporated Tragacanth Gum Hydrogel Biocomposite: A Promising Adsorbent for Crystal Violet Dye Removal from Aqueous Solutions" Gels 9, no. 12: 959. https://doi.org/10.3390/gels9120959