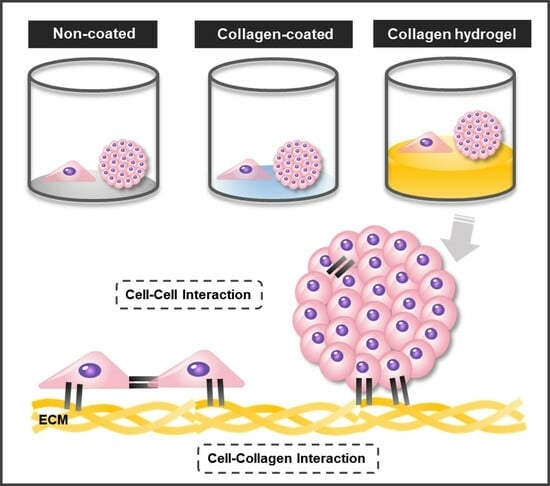
2.1. Characterization of Collagen Substrate
Three types of cell culture plates were prepared to investigate the combined influence of matrix stiffness and substrate composition on cell–matrix interactions. In the non-coated group, a commercially available cell culture well plate substrate was utilized. The collagen-coated group involved the application of collagen solution to the well plate to enhance cell–collagen interactions while maintaining the original stiffness of the substrate. In the collagen hydrogel group, a collagen hydrogel with a thickness of approximately 0.7 mm was formed in a well plate to create a soft substrate that promotes cell–collagen interactions (
Figure 1). A 2D monolayer culture was conducted by seeding NIH3T3 fibroblasts directly onto each prepared substrate. For a 3D spheroid culture, NIH3T3 fibroblast spheroids were formed and then seeded onto the substrate.
The collagen hydrogel was prepared by a crosslinking method using riboflavin phosphate (RFP) as a photosensitizer. This RFP-mediated collagen crosslinking method was used in a previous study and provides a convenient way to adjust the stiffness of the collagen hydrogel by varying the exposure time to blue light [
9]. In this study, the blue light exposure time was fixed at 10 min and no further experiments were performed to fine-tune the stiffness of the collagen hydrogel. However, the exposure time can be easily modified depending on the desired cell type and specific research objectives.
To evaluate the properties of the collagen hydrogel, the storage modulus (G′) and loss modulus (G″) were measured using a rheometer (
Figure 2). Frequency sweep rheology determines the relationship between G′ and G″ of a material at different frequencies, providing a rheological method to analyze viscoelastic properties and material state [
13]. Throughout the frequency range, G′ showed higher values than G″, indicating that the collagen hydrogel exhibited the behavior of gel.
Based on the successful formation of the collagen hydrogel, a comparative analysis was conducted on the characteristics of the three substrates. First, we measured the average roughness of each substrate (
Figure 3). The results obtained by calculating the standard deviation of the root mean square roughness (Rq) of the matrix surface indicated an increased surface roughness for the collagen-coated (32.78 ± 5.47) nm and the collagen hydrogel substrate (156.20 ± 5.64) nm compared to the non-coated substrate (0.14 ± 1.00) nm. The higher standard deviation for the non-coated substrate was attributed to surface contamination. The collagen hydrogel substrate had approximately five times the roughness of the collagen-coated substrate. It was evident that the non-coated surfaces maintained a flat and smooth appearance while the collagen-coated surfaces exhibited increased roughness due to the collagen coating. Although the collagen hydrogel showed a relatively small difference from the collagen-coated, it had the highest roughness of all the substrates, with all substrate roughness measurements falling in the nanometer range.
Cell–surface interactions play a critical role in cell attachment, proliferation, and tissue regeneration, and the surface topography of biomaterials significantly influences cell behavior and biocompatibility [
14]. Typically, surface roughness determines the surface area available for cell attachment and growth, thus influencing initial cell adhesion and interaction with the extracellular matrix. While the measured substrate roughness varied, they were all in the nanometer range and were small compared to the cell size. Therefore, we concluded that the roughness of the three substrates was not a significant factor in inducing substantial changes in cell behavior.
Another factor that can influence cell behavior is the wettability of the substrate surface. Substrate wettability is a critical factor in cell adhesion and determines cell spreading, migration, and proliferation [
15]. Measurement of the contact angle by water contact angle analysis revealed contact angles of 90.69 ± 0.64° for the non-coated substrate, 71.53 ± 1.99° for the collagen-coated substrate, and 56.87 ± 2.33° for the collagen hydrogel substrate (
Figure 4). Coating the surface with collagen reduced the contact angle due to the hydrophilicity of collagen. In addition, the contact angle of the collagen hydrogel, which contains water in the matrix, decreased even more compared to the collagen coating.
In general, cells adhere most effectively to surfaces with contact angles between 40 and 70°, and for fibroblast cells in particular, the highest adhesion strength is achieved when the contact angle is between 60 and 80° [
15,
16]. Overall, all surfaces provided contact angles within a range suitable for fibroblast cell attachment. Notably, the collagen-coated substrate theoretically provided the most favorable contact angle. By culturing cells on substrates with different contact angles, we established conditions ideal for examining cell–matrix interactions while considering both stiffness and wettability.
Three substrates, non-coated, collagen-coated, and collagen hydrogel, were characterized for their effect on cell–matrix interactions. The collagen hydrogel, prepared by riboflavin phosphate-mediated crosslinking, exhibited viscoelastic properties. Comparative analysis revealed increased surface roughness for coated and hydrogel substrates, with the latter having the highest roughness. Surface wettability varied but provided suitable conditions for fibroblast attachment. Furthermore, the chemical composition of the collagen-coated and collagen hydrogel substrates was the same (
Figure S1). These characterizations provide the basis for understanding the influence of the substrate on cell behavior in subsequent analyses.
2.2. Spheroid Behavior and Migration
A round bottom plate was coated with a coating solution and 2 × 10
3 NIH3T3 cells were seeded to form 3D spheroids. The size of the resulting spheroids and their migration over time were measured (
Figure 5). The initial diameter of the spheroids was 510 ± 44 μm. After the spheroids were transferred to each substrate, the average diameter and area of the spheroids were measured on days 1, 4, and 7. The average diameters and areas for spheroid migration on the non-coated, collagen-coated, and collagen hydrogel substrates on days 1, 4, and 7 are shown in
Table 1.
Diameter represents the distance that cells migrated from the center of the spheroid, while area was quantified using ImageJ software (version 1.52a) to measure the area where cells were attached in the images. In all groups, there was an increasing trend in the distance and area of cell migration from the center of the spheroid to the substrate surface over time. The spheroids on collagen hydrogel substrates showed the most extensive migration, followed by collagen-coated and non-coated substrates, which showed a gradual decrease in migration over time.
The surfaces of collagen-coated and collagen hydrogel substrates provide abundant cell–collagen interactions, suggesting that cell migration may be more active compared to the non-coated substrate. Collagen is a major protein component of the extracellular matrix (ECM) and plays a critical role in cell attachment and proliferation by regulating cytoskeletal rearrangement and signal transduction [
17]. In contrast, cells on the non-coated substrate were confined to a smaller area over time, reflecting slower cell migration due to reduced cell–matrix interaction on an untreated surface.
The observed differences in cell migration between the different substrates prompt a discussion on the interrelated influence of surface properties on cell behavior. The enhanced migration on collagen-coated and collagen hydrogel substrates, attributed to enriched cell–collagen interactions, is consistent with the well-established role of collagen in facilitating cell attachment and proliferation through cytoskeletal rearrangement and signal transduction mechanisms [
17]. In contrast, the slower migration on the non-coated substrate highlights the importance of surface treatment in promoting effective cell–matrix interactions.
Notably, the comparison between collagen-coated and collagen hydrogel substrates revealed a higher migration rate on the hydrogel despite similar collagen-based surfaces. This intriguing finding suggests that factors beyond surface composition, such as surface wettability and stiffness, contribute to the observed differences in cell behavior. While performing dedicated experiments to individually assess the effects of surface wettability and stiffness was challenging due to their interrelated nature, the collective effect of these surface properties on cell migration is evident from the observed patterns.
The significant influence of surface material composition, wettability, and stiffness collectively highlights the need for a holistic understanding of substrate properties in guiding cell behavior. This nuanced perspective is critical for the design of biomaterials in various biomedical applications, ranging from regenerative medicine to tissue engineering, where precise control of cell migration and interactions is paramount.
2.3. Evaluation of Cell Proliferation, Morphological Changes, and Extracellular Matrix Secretion
MTT assays were performed to evaluate cell proliferation on the three types of substrates (
Figure 6). In addition, to investigate the effects of cell–cell interaction, experiments were divided into two categories: 2D cell culture and 3D spheroid culture. Considering 2D and 3D culture conditions, cell proliferation on non-coated and collagen-coated substrates was more active in 2D culture than in 3D spheroid culture. In contrast, on collagen hydrogel substrates, 3D spheroid proliferation exceeded that of 2D culture.
When comparing proliferation rates between 2D and 3D spheroid cultures, 2D cultures generally showed more active proliferation. In 2D culture, cells are seeded as single cells, providing enough space to ensure low cell density upon cell attachment to the substrate. In 3D spheroid culture, cells are densely packed and proliferation occurs primarily as cells in the outermost layers of the spheroid migrate along the substrate. Thus, a proliferation of 2D cultured cells was more active than 3D spheroid culture on both non-coated and collagen-coated substrates. However, collagen hydrogel substrates showed the opposite result. Fibroblasts in 2D culture on collagen hydrogel substrates did not show robust proliferation compared to other substrates, but 3D spheroids on these substrates showed more active proliferation. As suggested by the migration results in
Figure 5, it can be inferred that the cells forming the spheroids on collagen hydrogel substrates migrated smoothly, resulting in more active proliferation than on other substrates.
In addition to cell proliferation, the secretion of collagen and glycosaminoglycans (GAGs) from the cells was measured to assess their functional performance (
Figure 7). Fibroblasts are cells that play an important role in the composition of the extracellular environment, with collagen and GAGs as their major secreted factors [
18,
19,
20]. To normalize the total collagen and GAGs secretion to the function of each cell, it was divided by the measured MTT values. Collagen secretion was most active in cells cultured on collagen hydrogel substrates, and there was little difference between 2D and 3D cultures. Notably, collagen secretion remained consistently high from day 1 to day 7 without a decrease. For the other substrates, non-coated and collagen-coated, cell seeding conditions rather than substrate type had a greater influence on collagen secretion. In all cases, 3D spheroid cultures showed higher collagen secretion than 2D cultures. However, in 3D culture conditions, collagen secretion was maintained until day 4 and then decreased significantly by day 7. In 2D culture conditions, there was a steady, low level of collagen secretion from day 1 to day 7.
The secretion of GAGs showed a decreasing trend over time in all groups, with almost indistinguishable secretion levels on day 7 in all groups. On day 1, cells cultured in 3D spheroids on collagen hydrogel substrates showed significantly higher secretion of GAGs. Subsequently, cells cultured in 3D spheroids on collagen-coated and non-coated substrates also showed higher secretion of GAGs. Cells cultured on collagen hydrogel in 2D culture conditions showed a GAGs secretion level similar to that of 3D spheroids cultured on non-coated substrates and higher than the other 2D cultures.
Except for 2D cultures on collagen hydrogel substrates, all groups showed higher collagen and GAG secretion in 3D spheroid cultures compared to 2D cultures. This suggests that cell–cell interactions play an important role in influencing fibroblast function in 3D spheroid cultures. The intercellular space in spheroids, where proteins such as fibronectin, collagen, and GAGs are secreted and accumulated, is known to be very active in 3D culture conditions [
21]. When fibroblasts were cultured on collagen hydrogel substrates, cells in the 2D culture conditions exhibited levels of function that were higher than or similar to those of 3D spheroids on other substrates. This suggests that while cell–cell interaction is critical, adequate cell–matrix interaction can sufficiently maintain cell functionality.
2.4. Immunofluorescence Analysis
Immunofluorescence analysis was used to evaluate cell morphology and expression to better understand the effects of cell–cell and cell–matrix interactions on cells. Cell morphology was visualized using phalloidin, which binds selectively to F-actin filaments (
Figure 8). Overall, a fibrous, linear structure was observed in all groups. In 2D cell culture conditions on non-coated and collagen-coated substrates, cells spread over a wide area, with F-actin appearing sharply linear. However, on collagen hydrogel substrates, cells were densely packed even in 2D culture conditions. In addition, F-actin expression was relatively low.
The central role of F-actin in shaping the cellular cytoskeleton and influencing cell structure, adhesion, and functions such as cell movement and muscle contraction is well established [
22]. Recent studies indicate that matrix stiffness plays a critical role in regulating the reorganization and morphological distribution of the cellular cytoskeleton, with a positive correlation observed between matrix stiffness and F-actin expression [
23,
24]. In the context of 2D culture on collagen hydrogel, which is characterized by a dense cell structure, an environment was created that promotes enhanced cell–cell interactions, providing valuable insights into the influence of matrix stiffness on cellular dynamics.
When staining by 3D spheroid culture, it was difficult to observe the central part of the spheroid due to the high cell density, so the focus was on the area extending from the center of the spheroid due to migration. On non-coated substrates, where cell migration was not active, a relatively high density of F-actin was observed. However, unlike what was seen on collagen hydrogel substrates, the cells were not clustered but were individually attached to the substrate, maintaining their spacing. Collagen-coated substrates showed a relatively lower cell density compared to non-coated substrates, but the overall results were similar. On collagen hydrogel substrates, in both 2D and 3D cultures, F-actin images were faint, and the cellular cytoskeleton was not clearly visible, with an overall tendency for cells to cluster.
In 3D spheroid culture, similar to 2D culture, it was observed that cells cultured on stiff substrates, such as non-coated and collagen-coated substrates, promoted fibrotic transformation mechanisms. This is characteristic of fibroblasts and the pressure exerted on the cells by the stiffness of the matrix affects the cells, increasing contractility and decreasing cell mobility [
11]. When comparing non-coated and collagen-coated substrates, it was evident that the cell cytoskeleton was influenced by both substrate stiffness and composition but showed a greater dependence on matrix stiffness.
To evaluate the influence of matrix stiffness and cell–cell interaction, the expression levels of Yes-associated protein (YAP) and E-cadherin were observed by immunostaining on day 7 (
Figure 9). In 2D cell culture, cells on non-coated substrates exhibited a general distribution of YAP and E-cadherin staining and showed the highest relative fluorescence intensity. In contrast, collagen-coated and collagen hydrogel substrates showed relatively narrow areas of fluorescence with significantly lower fluorescence intensity compared to non-coated substrates. Collagen-coated substrates showed a more extensive presence of YAP and E-cadherin compared to collagen hydrogel substrates, although the difference in fluorescence intensity of YAP was not observed, E-cadherin fluorescence intensity was slightly higher in collagen-coated substrates than in collagen hydrogel substrates.
YAP is a protein that senses various physical cues, including matrix stiffness and mechanical forces associated with cell structure and the cytoskeleton. It translates these cues into cell-specific transcriptional programs that induce gene expression [
25]. Cells induced by a stiff ECM require YAP function, whereas cells associated with a soft ECM require inactivation of YAP function, thereby suppressing YAP activity [
26]. E-cadherin is a core component of adherens junctions, which are essential for cell adhesion and maintenance of epithelial phenotypes. It anchors cells and promotes cell–cell interactions by physically restraining cell movement [
27]. In addition to promoting cell adhesion, E-cadherin initiates signaling that regulates cell shape, movement, proliferation, differentiation, and survival [
28].
In 2D cell culture, the observed increase in YAP activity was likely due to individual cells experiencing increased stiffness due to the rigid substrate. This is because stiff substrates transmit mechanical forces directly to the nucleus, resulting in nuclear flattening and increased YAP nuclear translocation [
26]. Since the non-coated substrate had no collagen treatment, cell–cell interactions were most likely due to the lack of collagen, which is why the epithelial marker E-cadherin was also expressed at high levels. Collagen hydrogel substrates had very low stiffness due to their soft matrix properties, which exerted minimal pressure on the cells, resulting in inhibition of YAP activity. YAP signaling was found to be responsive to mechanical transitions in cell shape, cytoskeletal integrity, and mechanical forces throughout the cell or tissue, and it is known that an increase in F-actin cytoskeletal assembly leads to increased nuclear YAP [
27]. This is consistent with the immunofluorescence results showing increased YAP activity on non-coated and collagen-coated substrates where the F-actin cytoskeleton was prominently displayed. Furthermore, although collagen matrix-cell interaction was active, cell–cell interaction was very weak, resulting in the suppression of E-cadherin expression. Collagen-coated substrates, which were similar to non-coated substrates in terms of substrate stiffness, had little effect on 2D cells, suggesting that the expression of YAP and E-cadherin in 2D cell culture is more associated with the presence of collagen.
In 3D spheroid culture, the prominent intensity of E-cadherin and YAP was observed in the center of the spheroid. This expression was primarily attributed to cell–cell interactions within the spheroid rather than cell–matrix interactions. As a result, differences in the distribution and intensity of E-cadherin and YAP between groups were not as pronounced as in the previous 2D culture. In the 2D culture, E-cadherin and YAP expression was highly localized and showed strong intensity, while within the spheroids, the expression was more evenly distributed throughout the spheroid rather than restricted to specific areas. In particular, the distribution of E-cadherin and YAP was concentrated in the inner regions of the spheroid rather than in the outer regions. Notably, the staining area of spheroids on collagen hydrogel substrates was narrower compared to the other two substrates.
In general, cells cultured at low density show reduced expression of the epithelial marker E-cadherin [
29]. Consequently, in 3D culture, strong E-cadherin expression was centered around the spheroid core, which had a high cell density and was rarely observed in 2D culture. YAP activity was also inhibited except in the spheroid center, where the low stiffness of the collagen and extensive cell movement resulted in minimal pressure, making it the most biologically relevant collagen substrate environment for 3D spheroid culture. The inhibitory effect of low mechanical stress on YAP activity has been well documented [
30]. Thus, it was confirmed that YAP and E-cadherin expressions are primarily influenced by cell density and cell–cell interactions, with secondary effects from cell–matrix interactions.
This research provided insight into the key factors influencing fibroblast behavior under 2D and 3D culture conditions and the influence of substrate type. The most significant factor identified was cell–cell interaction in 3D spheroid culture, which minimized the effect of substrate and maintained fibroblast functionality. Substrate stiffness had a significant effect, with the soft collagen hydrogel substrate significantly improving fibroblast function compared to stiff substrates. Of particular significance, fibroblasts cultured on collagen hydrogel substrates in a 2D environment exhibit similar cell functionality as those cultured in 3D. Surface composition had the least effect, with collagen-coated surfaces showing no significant differences from non-coated surfaces in all experiments. The research elucidates the impact of cell–cell and cell–matrix interactions, as well as stiffness, on cellular behavior. This has significant future implications in regenerative medicine, including tissue engineering and drug screening, where cellular approaches play a crucial role.