Polymeric Hydrogels for Intervertebral Disc Replacement/Integration: Playing with the Chemical Composition for Tuning Shear Behavior and Hydrophilicity
Abstract
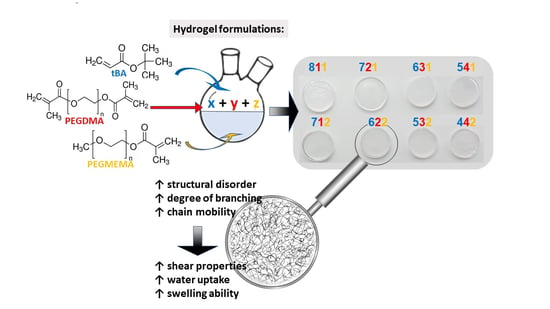
:1. Introduction
2. Results and Discussion
2.1. Water Uptake/Swelling Behavior
2.2. DSC Measurements
2.3. Dynamic Shear Measurements
2.4. Solid-State Nuclear Magnetic Resonance Study
2.5. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of tBA/PEGDMA/PEGMEMA Samples
4.3. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stranding, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 40th ed.; Elsevier: Amsterdam, The Netherland, 2016; pp. 712–718. ISBN 9780120885480. [Google Scholar]
- Galbusera, F.; Wilke, H.J. Biomechanics of the Spine: Basic Concepts, Spinal Disorders and Treatments; Academic Press: London, UK, 2018; ISBN 978-0-12-812851-0. [Google Scholar]
- Wei, Q.; Zhang, X.; Zhou, C.; Ren, Q.; Zhang, Y. Roles of large aggregating proteoglycans in human intervertebral disc degeneration. Connect. Tissue Res. 2019, 60, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, M.; Wade, K.; Haj-Ali, R. The Mechanical Role of Collagen Fibers in the Intervertebral Disc; Elsevier Ltd.: Amsterdam, The Netherland, 2018. [Google Scholar] [CrossRef]
- Acaroglu, E.R.; Iatridis, J.C.; Setton, L.A.; Foster, R.J.; Mow, V.C.; Wedenbaum, M. Degeneration and aging affect the tensile behaviour of human lumbar anulus fibrosus. Spine 1995, 20, 2690–2701. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Mayoux-Benhamou, M.A.; Poiraudeau, S.; Revel, M. Anatomy, biology, physiology, and biomechanics of intervertebral disk and other anatomical structures of the lumbar spine. EMC-Rhumatol.-Orthop. 2004, 1, 487–507. [Google Scholar] [CrossRef]
- Pattappa, G.; Li, Z.; Peroglio, M.; Wismer, N.; Alini, M.; Grad, S. Diversity of intervertebral disc cells: Phenotype and function. J. Anat. 2012, 221, 480–496. [Google Scholar] [CrossRef]
- Hwang, P.Y.; Chen, J.; Jing, L.; Hoffman, B.D.; Setton, L.A. The role of extracellular matrix elasticity and composition in regulating the nucleus pulposus cell phenotype in the intervertebral disc: A narrative review. J. Biomech. Eng. 2014, 136, 021010. [Google Scholar] [CrossRef]
- Newell, N.; Little, J.P.; Christou, A.; Adams, M.A.; Adam, C.J.; Masouros, S.D. Biomechanics of the human intervertebral disc: A review of testing techniques and results. J. Mech. Behav. Biomed. Mater. 2017, 69, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Marchand, F.; Ahmed, A.M. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 1990, 15, 402–410. [Google Scholar] [CrossRef]
- O’Connell, G.D.; Sen, S.; Elliott, D.M. Human annulus fibrosus material properties from biaxial testing and constitutive modeling are altered with degeneration. Biomech. Model. Mechanobiol. 2012, 11, 493–503. [Google Scholar] [CrossRef]
- Cassidy, J.J.; Hiltner, A.; Baer, E. Hierarchical structure of the intervertebral disc. Connect. Tissue Res. 1989, 23, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Müller-Gerbl, M.; Weißer, S.; Linsenmeier, U. The distribution of mineral density in the cervical vertebral endplates. Eur. Spine J. 2008, 17, 432–438. [Google Scholar] [CrossRef]
- Malandrino, A.; Lacroix, D.; Hellmich, C.; Ito, K.; Ferguson, S.J.; Noailly, J. The role of endplate poromechanical properties on the nutrient availability in the intervertebral disc. Osteoarthr. Cartil. 2014, 22, 1053–1060. [Google Scholar] [CrossRef]
- Grunhagen, T.; Wilde, G.; Soukane, D.M.; Shirazi-Adl, S.A.; Urban, J.P. Nutrient Supply and Intervertebral Disc Metabolism. J. Bone Jt. Surg. Am. 2006, 88, 30–36. [Google Scholar]
- Rohlmann, A.; Pohl, D.; Bender, A.; Graichen, F.; Dymke, J.; Schmidt, H.; Bergmann, G. Activities of everyday life with high spinal loads. PLoS ONE 2014, 9, e98510. [Google Scholar] [CrossRef]
- Singha, K. Biomechanism Profile of Intervertebral Discs (IVD): Strategies to Successful Tissue Engineering for Spinal Healing by Reinforced Composite Structure. J. Tissue Sci. Eng. 2012, 3, 118. [Google Scholar] [CrossRef]
- Long, R.G.; Torre, O.M.; Hom, W.W.; Assael, D.J.; Iatridis, J.C. Design requirements for annulus fibrosus repair: Review of forces, displacements, and material properties of the intervertebral disk and a summary of candidate hydrogels for repair. J. Biomech. Eng. 2016, 138, 021007. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Thareja, P. Hydrogels differentiated by length scales: A review of biopolymer-based hydrogel preparation methods, characterization techniques, and targeted applications. Eur. Polym. J. 2022, 163, 110935. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Xuan, Y.; Zhang, S. Synthesis and Applications of Carboxymethyl Cellulose Hydrogels. Gels 2022, 8, 529. [Google Scholar] [CrossRef]
- Da Silva Baptista, J.; De Vasconcellos Fontes, R.B.; Liberti, E.A. Aging and degeneration of the intervertebral disc: Review of basic science. Coluna/Columna 2015, 14, 144–148. [Google Scholar] [CrossRef]
- Ito, K.; Creemers, L. Mechanisms of Intervertebral Disk Degeneration/Injury and Pain: A Review. Glob. Spine J. 2013, 3, 145–151. [Google Scholar] [CrossRef]
- Thompson, J.P.; Pearce, R.H.; Schechter, M.T.; Adams, M.E.; Tsang, I.K.; Bishop, P.B. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine 1990, 15, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Krasowska, M.; Ge, W.; Platts, K.; Marina, P.F.; Blencowe, A. Combining thermosensitive physical self-assembly and covalent cycloaddition chemistry as simultaneous dual cross-linking mechanisms for the preparation of injectable hydrogels with tuneable properties. Eur. Polym. J. 2023, 183, 111761. [Google Scholar] [CrossRef]
- Hwang, J.W.; Noh, S.M.; Kim, B.; Jung, H.W. Gelation and crosslinking characteristics of photopolymerized poly(ethylene glycol) hydrogels. J. Appl. Polym. Sci. 2015, 132, 1–6. [Google Scholar] [CrossRef]
- Van Uden, S.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Current strategies for treatment of intervertebral disc degeneration: Substitution and regeneration possibilities. Biomater. Res. 2017, 21, 22. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Arun Kumar, R.; Vishnu Priya, M.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Yom-Tov, O.; Seliktar, D.; Bianco-Peled, H. PEG-Thiol based hydrogels with controllable properties. Eur. Polym. J. 2016, 74, 1–12. [Google Scholar] [CrossRef]
- Schizas, C.; Kulik, G.; Kosmopoulos, V. Disc degeneration: Current surgical options. Eur. Cells Mater. 2010, 20, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Kalson, N.S.; Richardson, S.; Hoyland, J.A. Strategies for regeneration of the intervertebral disc. Regen. Med. 2008, 3, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Landi, A. Elastic resistance of the spine: Why does motion preservation surgery almost fail? World J. Clin. Cases 2013, 1, 134. [Google Scholar] [CrossRef]
- Williams, D. Benefit and risk in tissue engineering. Mater. Today 2004, 7, 24–29. [Google Scholar] [CrossRef]
- Tan, X.; Jain, E.; Barcellona, M.N.; Morris, E.; Neal, S.; Gupta, M.C.; Buchowski, J.M.; Kelly, M.; Setton, L.A.; Huebsch, N. Integrin and syndecan binding peptide-conjugated alginate hydrogel for modulation of nucleus pulposus cell phenotype. Biomaterials 2021, 277, 121113. [Google Scholar] [CrossRef]
- Meyer, U. The history of tissue engineering and regenerative medicine in perspective. In Fundamentals of Tissue Engineering and Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 5–12. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Barcellona, M.N.; Speer, J.E.; Fearing, B.V.; Jing, L.; Pathak, A.; Gupta, M.C.; Buchowski, J.M.; Kelly, M.; Setton, L.A. Control of adhesive ligand density for modulation of nucleus pulposus cell phenotype. Biomaterials 2020, 250, 120057. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef]
- Rana, D.; Kumar, T.S.; Ramalingam, M. Cell-Laden Hydrogels for Tissue Engineering. J. Biomater. Tissue Eng. 2014, 4, 507–535. [Google Scholar] [CrossRef]
- Peters, J.T.; Wechsler, M.E.; Peppas, N.A. Advanced biomedical hydrogels: Molecular architecture and its impact on medical applications. Regen. Biomater. 2021, 8, rbab060. [Google Scholar] [CrossRef]
- Hoti, G.; Caldera, F.; Cecone, C.; Pedrazzo, A.R.; Anceschi, A.; Appleton, S.L.; Monfared, Y.K.; Trotta, F. Effect of the cross-linking density on the swelling and rheological behavior of ester-bridged β-cyclodextrin nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef]
- Kolodziejski, W.; Klinowski, J. Kinetics of cross-polarization in solid-state NMR: A guide for chemists. Chem. Rev. 2002, 102, 613–628. [Google Scholar] [CrossRef]
- Voelkel, R. High-Resolution Solid-state 13C-NMR Spectroscopy of Polymers. Angew. Chem. 1988, 27, 1468–1483. [Google Scholar]
- Shapiro, Y.E. Structure and dynamics of hydrogels and organogels: An NMR spectroscopy approach. Prog. Polym. Sci. 2011, 36, 1184–1253. [Google Scholar] [CrossRef]
- Tang, B.; Yang, Z.; Zhang, S. Poly(polyethylene glycol methyl ether methacrylate) as novel solid-solid phase change material for thermal energy storage. J. Appl. Polym. Sci. 2012, 125, 1377–1381. [Google Scholar] [CrossRef]
- Bujak, P.; Henzel, N.; Matlengiewicz, M. Microstructure study of poly(tert-butyl acrylate) by 13C NMR spectroscopy. Int. J. Polym. Anal. Charact. 2007, 12, 431–443. [Google Scholar] [CrossRef]
- Castrignolles, P.; Graf, R.; Parkinson, M.; Wilhelm, M.; Gaborieau, M. Detection and quantification of branching in polyacrylates by size-exclusion chromatography (SEC) and melt-state 13C NMR spectroscopy. Polymer 2009, 50, 2373–2383. [Google Scholar] [CrossRef]
- Ahmad, N.M.; Heatley, F.; Lovell, P.A. Chain transfer to polymer in free-radical solution polymerization of n-butyl acrylate studied by NMR spectroscopy. Macromolecules 1998, 31, 2822–2827. [Google Scholar] [CrossRef]
- Barrenetxe, M.; Agirre, A.; Santos, J.I.; Badía, A.; Leiza, J.R.; Barquero, A. Oil-Based versus Bio-Based C8 Alkyl Chain (Meth)Acrylates in Emulsion Polymerization: Kinetics and Microstructure. Macromol. React. Eng. 2022, 16, 2200014. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Dirè, S.; Masneri, V.; Rovera, D.; Di Credico, B.; Callone, E.; Mascotto, S.; Pegoretti, A.; Ziarelli, F.; Scotti, R. Tailoring the Dielectric and Mechanical Properties of Polybutadiene Nanocomposites by Using Designed Ladder-like Polysilsesquioxanes. ACS Appl. Nano Mater. 2018, 1, 3817–3828. [Google Scholar] [CrossRef]
- Da Silva, E.P.; Tavares, M.I.B. Solid state NMR study of poly (methyl methacrylate)/polyvinylpyrrolidone blends. Polym. Bull. 1998, 41, 307–310. [Google Scholar] [CrossRef]
- Phinnyocheep, P.; Saelao, J.; Buzarè, J.Y. Mechanical properties; morphology and molecular characteristics of poly(ethylene terephthalate) toughened by natural rubber. Polymer 2007, 48, 5702–5712. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, M.; Kong, X.; Severtson, S.J.; Wang, W.J. Tailoring chain structures of l-lactide and ϵ-caprolactone copolyester macromonomers using: Rac-binaphthyl-diyl hydrogen phosphate-catalyzed ring-opening copolymerization with monomer addition strategy. RSC Adv. 2017, 7, 28661–28669. [Google Scholar] [CrossRef]
- Macdougall, L.J.; Pérez-Madrigal, M.M.; Shaw, J.E.; Worch, J.C.; Sammon, C.; Richardson, S.M.; Dove, A.P. Using Stereochemistry to Control Mechanical Properties in Thiol–Yne Click-Hydrogels. Angew. Chem.-Int. Ed. 2021, 60, 25856–25864. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Baig, C. Role of short chain branching in polymer structure and dynamics. J. Chem. Phys. 2016, 144, 081101. [Google Scholar] [CrossRef] [PubMed]
- Corneillie, S.; Smet, M. PLA architectures: The role of branching. Polym. Chem. 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Dirè, S.; Callone, E.; Ceccato, R.; Parrino, F.; Di Credico, B.; Mostoni, S.; Scotti, R.; D’Arienzo, M. Structural effects of TiO2 nanoparticles in photocurable ladder-like polysilsesquioxane nanocomposites. J. Sol-Gel Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Kumar, S.; Foster, R.J.; Weidenbaum, M.; Mow, V.C. Shear mechanical properties of human lumbar annulus fibrosus. J. Orthop. Res. 2005, 17, 732–737. [Google Scholar] [CrossRef]








| Sample Label | Nominal Composition, wt % | ||
|---|---|---|---|
| tBA | PEGDMA | PEGMEMA | |
| 811 | 80 | 10 | 10 |
| 721 | 70 | 20 | 10 |
| 631 | 60 | 30 | 10 |
| 541 | 50 | 40 | 10 |
| 712 | 70 | 10 | 20 |
| 622 | 60 | 20 | 20 |
| 532 | 50 | 30 | 20 |
| 442 | 40 | 40 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniglio, D.; Bissoli, E.; Callone, E.; Dirè, S.; Motta, A. Polymeric Hydrogels for Intervertebral Disc Replacement/Integration: Playing with the Chemical Composition for Tuning Shear Behavior and Hydrophilicity. Gels 2023, 9, 912. https://doi.org/10.3390/gels9110912
Maniglio D, Bissoli E, Callone E, Dirè S, Motta A. Polymeric Hydrogels for Intervertebral Disc Replacement/Integration: Playing with the Chemical Composition for Tuning Shear Behavior and Hydrophilicity. Gels. 2023; 9(11):912. https://doi.org/10.3390/gels9110912
Chicago/Turabian StyleManiglio, Devid, Elia Bissoli, Emanuela Callone, Sandra Dirè, and Antonella Motta. 2023. "Polymeric Hydrogels for Intervertebral Disc Replacement/Integration: Playing with the Chemical Composition for Tuning Shear Behavior and Hydrophilicity" Gels 9, no. 11: 912. https://doi.org/10.3390/gels9110912