Double-Network Hydrogel Films Based on Cellulose Derivatives and κ-Carrageenan with Enhanced Mechanical Strength and Superabsorbent Properties
Abstract
:1. Introduction
2. Results and Discussion
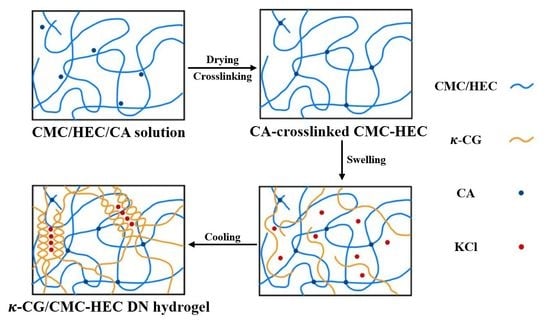
2.1. Preparation and Physicochemical Characterization of the CMC–HEC Hydrogels
2.2. Water-Absorbency Properties of the As-Prepared Dried DN Hydrogels
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Preparation of DN Hydrogels
4.3. Characterization
Author Contributions
Funding
Conflicts of Interest
References
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, H.; Lu, W.; Nishinari, K.; Fang, Y. New insights into food hydrogels with reinforced mechanical properties: A review on innovative strategies. Adv. Colloid Interface Sci. 2020, 285, 102278. [Google Scholar] [CrossRef]
- Yang, M.; Wu, J.; Graham, G.M.; Lin, J.; Huang, M. Hotspots, frontiers, and emerging trends of superabsorbent polymer research: A comprehensive review. Front. Chem. 2021, 9, 688127. [Google Scholar] [CrossRef]
- Yu, F.; Yang, P.; Yang, Z.; Zhang, X.; Ma, J. Double-network hydrogel adsorbents for environmental applications. Chem. Eng. J. 2021, 426, 131900. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldana, A.A.; Houben, S.; Moroni, L.; Baker, M.B.; Pitet, L.M. Trends in Double networks as bioprintable and injectable hydrogel scaffolds for tissue regeneration. ACS Biomater. Sci. Eng. 2021, 7, 4077–4101. [Google Scholar] [CrossRef]
- Xin, H. Double-network tough hydrogels: A brief review on achievements and challenges. Gels 2022, 8, 247. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Maiti, C.; Imani, K.B.C.; Jinhwan Yoon, J. Recent Advances in Design Strategies for Tough and Stretchable Hydrogels. ChemPlusChem 2021, 86, 601–611. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Lu, X.; Chan, C.Y.; Lee, K.I.; Ng, P.F.; Fei, B.; Xin, J.H.; Fu, J. Super-tough and thermo-healable hydrogel—Promising for shape-memory absorbent fiber. J. Mater. Chem. B 2014, 2, 7631–7638. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L. Recoverable and self-healing double network hydrogel based on κ-carrageenan. ACS Appl. Mater. Interfaces 2016, 8, 29749–29758. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L. Ultrastretchable and self-healing double-network hydrogel for 3D printing and strain sensor. ACS Appl. Mater. Interfaces 2017, 9, 26429–26437. [Google Scholar] [CrossRef] [PubMed]
- Exon, J.H. A review of the toxicology of acrylamide. J. Toxicol. Environ. Health B Crit. Rev. 2006, 9, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Synthesis and application of new temperature-responsive hydrogels based on carboxymethyl and hydroxyethyl cellulose derivatives for the functional finishing of cotton knitwear. Carbohydr. Polym. 2011, 85, 664–673. [Google Scholar] [CrossRef]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 2019, 88, 68–74. [Google Scholar] [CrossRef]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; El Achaby, M. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surface Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- Das, D.; Prakash, P.; Rout, P.K.; Bhaladhare, S. Synthesis and characterization of superabsorbent cellulose-based hydrogel for agriculture application. Starch-Stärke 2021, 73, 1900284. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.; Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Uyanga, K.A.; Okpozo, O.P.; Onyekwere, O.S.; Daoud, W.A. Citric acid crosslinked natural bi-polymer-based composite hydrogels: Effect of polymer ratio and beta-cyclodextrin on hydrogel microstructure. React. Funct. Polym. 2020, 154, 104682. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid crosslinked beta-cyclodextrin/ carboxymethylcellulose hydrogel films for controlled delivery of poorly soluble drugs. Carbohydr. Polym. 2017, 164, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Abou-Yousef, H.; Kamel, S. High efficiency antimicrobial cellulose-based nanocomposite hydrogels. J. Appl. Polym. Sci. 2015, 132, 42327. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Nishat, N.; Jadoun, S.; Ansari, M.Z. Polysaccharide based superabsorbent hydrogels and their methods of synthesis: A review. Carbohydr. Polym. Technol. Appl. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, C.; Kim, Y.; Jung, S. Dual crosslinked carboxymethyl cellulose/polyacrylamide interpenetrating hydrogels with highly enhanced mechanical strength and superabsorbent properties. Eur. Polym. J. 2020, 127, 109586. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; Carvalho, S.M.; Mansur, L.L.; Ramos, C.P.; Lage, A.P.; Mansur, H.S. Physicochemical properties and antimicrobial activity of biocompatible carboxymethylcellulose-silver nanoparticle hybrids for wound dressing and epidermal repair. J. Appl. Polym. Sci. 2018, 135, 45812. [Google Scholar] [CrossRef]
- Nakajima, T.; Kurokawa, T.; Ahmed, S.; Wu, W.; Gong, J.P. Characterization of internal fracture process of double network hydrogels under uniaxial elongation. Soft Matter 2013, 9, 1955–1966. [Google Scholar] [CrossRef]
- Kang, J.; Yun, S.I. Fmoc-phenylalanine as a building block for hybrid double network hydrogels with enhanced mechanical properties, self-recovery, and shape memory capability. Polymer 2022, 255, 125145. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Huang, L.; Chen, H.; Xu, K.; Tan, Y.; Wang, P.; Zheng, J. Fracture of the physically cross-linked first network in hybrid double network hydrogels. Macromolecules 2014, 47, 2140–2148. [Google Scholar] [CrossRef]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and equilibrium models of adsorption. In Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Springer: Cham, Switzerland, 2015; pp. 33–69. [Google Scholar]
- Lima, E.C.; Sher, F.; Guleria, A.; Saeb, M.R.; Anastopoulos, I.; Tran, H.N.; Hosseini-Bandegharaei, A. Is one performing the treatment data of adsorption kinetics correctly? J. Environ. Chem. Eng. 2021, 9, 104813. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kang, J.; Yun, S.I. Alginate-reinforced poly (3-hydroxybutyrate)/poly (hydroxybutyrate-co-hydroxyvalerate) aerogel monoliths fabricated by phase separation as environmental floating adsorbents. Int. J. Biol. Macromol. 2022, 217, 956–968. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Kim, H.; Kim, J.; Kim, D. Enhancement of gel strength of itaconic acid-based superabsorbent polymer composites using oxidized starch. Polymers 2021, 13, 2859. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, E.; Motesharezedeh, B.; Shirinfekr, A.; Samar, S.M. Synthesis and swelling behavior of environmentally friendly starch-based superabsorbent hydrogels reinforced with natural char nano/micro particles. J. Environ. Chem. Eng. 2020, 8, 103583. [Google Scholar] [CrossRef]










| Sample | A3383/A889 | A1722/A889 | A1230/A889 |
|---|---|---|---|
| CMC/HEC | 0.61 | 0.20 | 0.26 |
| CA-crosslinked CMC–HEC | 0.37 | 7.45 | 0.39 |
| κ-CG/ CMC–HEC | Film | Particle | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (exp.) | PFO | PSO | qm (exp.) | PFO | PSO | |||||||||
| qm | k1 | R2 | qm | k2 | R2 | qm | k1 | R2 | qm | k2 | R2 | |||
| 0/1 | 5277 | 4390 | 0.010 | 0.8967 | 5294 | 6.6 × 10−6 | 0.9996 | 6648 | 6149 | 0.021 | 0.8084 | 6711 | 6.2 × 10−6 | 0.9998 |
| 1/25 | 5097 | 3932 | 0.012 | 0.8364 | 5432 | 7.6 × 10−6 | 0.9998 | 6139 | 4784 | 0.086 | 0.7848 | 6177 | 6.8 × 10−6 | 0.9996 |
| 1/10 | 4808 | 3782 | 0.015 | 0.9106 | 4847 | 1.0 × 10−5 | 0.9999 | 5751 | 4260 | 0.008 | 0.7646 | 5760 | 8.5 × 10−6 | 0.9994 |
| 1/7 | 4393 | 3002 | 0.015 | 0.8298 | 4429 | 1.8 × 10−5 | 0.9999 | 5256 | 4000 | 0.011 | 0.6940 | 5291 | 9.9 × 10−6 | 0.9997 |
| 1/5 | 4649 | 3782 | 0.013 | 0.8791 | 4735 | 8.1 × 10−6 | 0.9997 | 4812 | 3630 | 0.009 | 0.7545 | 4857 | 7.5 × 10−6 | 0.9994 |
| κ-CG/ CMC–HEC | Film | Particle | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (exp.) | PFO | PSO | qm (exp.) | PFO | PSO | |||||||||
| qm | k1 | R2 | qm | k2 | R2 | qm | k1 | R2 | qm | k2 | R2 | |||
| 0/1 | 5277 | 5067 | 0.027 | 0.9971 | 5400 | 6.4 × 10−6 | 0.9956 | 6648 | 6284 | 0.030 | 0.9860 | 6673 | 6.2 × 10−6 | 0.9982 |
| 1/25 | 5097 | 4929 | 0.034 | 0.9978 | 5228 | 8.8 × 10−6 | 0.9924 | 6139 | 5727 | 0.033 | 0.9818 | 6078 | 7.3 × 10−6 | 0.9982 |
| 1/10 | 4808 | 4712 | 0.035 | 0.9990 | 4994 | 9.5 × 10−6 | 0.9922 | 5751 | 5297 | 0.041 | 0.9731 | 5638 | 9.6 × 10−6 | 0.9924 |
| 1/7 | 4393 | 4288 | 0.052 | 0.9954 | 4545 | 1.5 × 10−5 | 0.9938 | 5256 | 4960 | 0.040 | 0.9842 | 5267 | 1.0 × 10−5 | 0.9986 |
| 1/5 | 4649 | 4586 | 0.029 | 0.9967 | 4871 | 7.8 × 10−6 | 0.9876 | 4812 | 4494 | 0.038 | 0.9872 | 4796 | 1.0× 10−5 | 0.9905 |
| κ-CG/ CMC–HEC | Particle (pH 3) | Particle (pH 11) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (exp.) | PFO | PSO | qm (exp.) | PFO | PSO | |||||||||
| qm | k1 | R2 | qm | k2 | R2 | qm | k1 | R2 | qm | k2 | R2 | |||
| 0/1 | 4335 | 4205 | 0.043 | 0.9808 | 4437 | 1.4 × 10−5 | 0.9908 | 7079 | 6798 | 0.013 | 0.9641 | 8643 | 1.5 × 10−6 | 0.9766 |
| 1/25 | 4100 | 3911 | 0.047 | 0.9864 | 4141 | 1.5 × 10−5 | 0.9955 | 7113 | 6698 | 0.015 | 0.9090 | 7896 | 2.4 × 10−6 | 0.9351 |
| 1/7 | 3898 | 3659 | 0.050 | 0.9843 | 3885 | 1.8 × 10−5 | 0.9988 | 7009 | 6535 | 0.017 | 0.9526 | 7962 | 2.3 × 10−6 | 0.9705 |
| 1/5 | 3884 | 3761 | 0.049 | 0.9835 | 3983 | 1.7 × 10−5 | 0.9889 | 7178 | 6405 | 0.020 | 0.9033 | 7385 | 3.6 × 10−6 | 0.9389 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Yun, S.I. Double-Network Hydrogel Films Based on Cellulose Derivatives and κ-Carrageenan with Enhanced Mechanical Strength and Superabsorbent Properties. Gels 2023, 9, 20. https://doi.org/10.3390/gels9010020
Kang J, Yun SI. Double-Network Hydrogel Films Based on Cellulose Derivatives and κ-Carrageenan with Enhanced Mechanical Strength and Superabsorbent Properties. Gels. 2023; 9(1):20. https://doi.org/10.3390/gels9010020
Chicago/Turabian StyleKang, Jiseon, and Seok Il Yun. 2023. "Double-Network Hydrogel Films Based on Cellulose Derivatives and κ-Carrageenan with Enhanced Mechanical Strength and Superabsorbent Properties" Gels 9, no. 1: 20. https://doi.org/10.3390/gels9010020