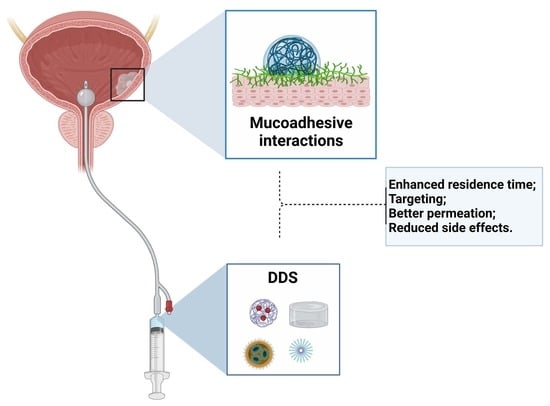
Mucoadhesive Polymers and Their Applications in Drug Delivery Systems for the Treatment of Bladder Cancer
Abstract
:1. Overview
2. Fundamentals
2.1. Mucosa Structure

2.2. Available Treatments
2.3. Drug Delivery Systems (DDS)
2.4. Adhesion/Bioadhesion/Mucoadhesion
3. Mucoadhesive Polymeric Drug Delivery Systems
3.1. Theories and Mechanisms
3.1.1. Electronic Theory
3.1.2. Wetting Theory
3.1.3. Adsorption Theory
3.1.4. Diffusion Theory
3.1.5. Mechanical Theory
3.1.6. Cohesive or Fracture Theory
3.2. Methods to Evaluate Mucoadhesivity of Polymers
3.2.1. In Vitro and Ex Vivo Methods
Tensile Strength
Shear Strength
Rheological Methods
Washability Test
Colloidal Gold Staining Method
Mechanical Spectroscopic Method
Falling Liquid Film Method
Biacore System
Confocal Laser Scanning Microscopy (CLSM) Method
3.2.2. In Vivo Methods
3.3. Mucoadhesive Polymers Suitable for the Development of DDs for Bladder Cancer Treatment
3.4. In Situ Gelling Polymers
3.4.1. Thermo-Responsive Systems
3.4.2. pH-Responsive Systems
3.4.3. Ionic-Responsive Systems
3.4.4. In Situ Gelation Triggered by Genipin
3.5. Rheological Aspects
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer; GLOBOCAN Global Bladder Cancer Statistics. 2020, pp. 1–2. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf (accessed on 9 September 2022).
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The Global Burden of Urinary Bladder Cancer: An Update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Degeorge, K.C.; Holt, H.R.; Hodges, S.C. Bladder Cancer: Diagnosis and Treatment. Am. Fam. Physician 2017, 96. [Google Scholar]
- Cassell, A.; Yunusa, B.; Jalloh, M.; Mbodji, M.M.; Diallo, A.; Ndoye, M.; Diallo, Y.; Labou, I.; Niang, L.; Gueye, S.M. Non-Muscle Invasive Bladder Cancer: A Review of the Current Trend in Africa. World J. Oncol. 2019, 10, 123–131. [Google Scholar] [CrossRef]
- Martinez Rodriguez, R.H.; Buisan Rueda, O.; Ibarz, L. Bladder Cancer: Present and Future. Med. Clin. (Barc). 2017, 149, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Down, C.J.; Nair, R.; Thurairaja, R. Bladder Cancer. Surgery 2016, 34, 532–539. [Google Scholar] [CrossRef]
- Jain, P.; Kathuria, H.; Momin, M. Clinical Therapies and Nano Drug Delivery Systems for Urinary Bladder Cancer. Pharmacol. Ther. 2021, 226. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder Cancer a Review. JAMA - J. Am. Med. Assoc. 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Lemon, G.; Hilborn, J.; Chronakis, I.S.; Fossum, M. Bladder Biomechanics and the Use of Scaffolds for Regenerative Medicine in the Urinary Bladder. Nat. Rev. Urol. 2018, 15, 155–174. [Google Scholar] [CrossRef]
- Fry, C.H.; Vahabi, B. The Role of the Mucosa in Normal and Abnormal Bladder Function. Basic Clin. Pharmacol. Toxicol. 2016, 119, 57–62. [Google Scholar] [CrossRef]
- Wang, S.; Jin, S.; Shu, Q.; Wu, S. Strategies to Get Drugs across Bladder Penetrating Barriers for Improving Bladder Cancer Therapy. Pharmaceutics 2021, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder Cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Care, P. Bladder Cancer: Diagnosis and Management of Bladder Cancer: © NICE (2015) Bladder Cancer: Diagnosis and Management of Bladder Cancer. BJU Int. 2017, 120, 755–765. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Mostafid, H.; Khutoryanskiy, V.V. Advances in Intravesical Drug Delivery Systems to Treat Bladder Cancer. Int. J. Pharm. 2017, 532, 105–117. [Google Scholar] [CrossRef] [PubMed]
- GuhaSarkar, S.; Banerjee, R. Intravesical Drug Delivery: Challenges, Current Status, Opportunities and Novel Strategies. J. Control. Release 2010, 148, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.Y.C.; Wang, H. Introduction for Design of Nanoparticle Based Drug Delivery Systems. Curr. Pharm. Des. 2016, 23. [Google Scholar] [CrossRef]
- Shende, P.; Basarkar, V. Recent Trends and Advances in Microbe-Based Drug Delivery Systems. DARU, J. Pharm. Sci. 2019, 27, 799–809. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in Drug Delivery Systems: Work in Progress Still Needed? Int. J. Pharm. X 2020, 2, 100050. [Google Scholar] [CrossRef]
- Sharif, S.; Abbas, G.; Hanif, M.; Bernkop-Schnürch, A.; Jalil, A.; Yaqoob, M. Mucoadhesive Micro-Composites: Chitosan Coated Halloysite Nanotubes for Sustained Drug Delivery. Colloids Surfaces B Biointerfaces 2019, 184. [Google Scholar] [CrossRef]
- Lian, Z.; Ji, T. Functional Peptide-Based Drug Delivery Systems. J. Mater. Chem. B 2020, 8, 6517–6529. [Google Scholar] [CrossRef]
- Lupo, N.; Jalil, A.; Nazir, I.; Gust, R.; Bernkop-Schnürch, A. In Vitro Evaluation of Intravesical Mucoadhesive Self-Emulsifying Drug Delivery Systems. Int. J. Pharm. 2019, 564, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Stærk, K.; Hjelmager, J.S.; Alm, M.; Thomsen, P.; Andersen, T.E. A New Catheter-Integrated Drug-Delivery System for Controlled Intravesical Mitomycin C Release. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 409.e19–409.e26. [Google Scholar] [CrossRef]
- Kaldybekov, D.B.; Tonglairoum, P.; Opanasopit, P.; Khutoryanskiy, V.V. Mucoadhesive Maleimide-Functionalised Liposomes for Drug Delivery to Urinary Bladder. Eur. J. Pharm. Sci. 2018, 111, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nikezić, A.V.V.; Bondžić, A.M.; Vasić, V.M. Drug Delivery Systems Based on Nanoparticles and Related Nanostructures. Eur. J. Pharm. Sci. 2020, 151. [Google Scholar] [CrossRef] [PubMed]
- Colone, M.; Calcabrini, A.; Stringaro, A. Drug Delivery Systems of Natural Products in Oncology. Molecules 2020, 25, 4560. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, K.; Wang, J.; Zhao, R.; Zhang, Q.; Kong, X. Poly(Amidoamine)-Modified Mesoporous Silica Nanoparticles as a Mucoadhesive Drug Delivery System for Potential Bladder Cancer Therapy. Colloids Surf. B Biointerfaces 2020, 189, 110832. [Google Scholar] [CrossRef]
- Gonzaga, R.V.; do Nascimento, L.A.; Santos, S.S.; Machado Sanches, B.A.; Giarolla, J.; Ferreira, E.I. Perspectives About Self-Immolative Drug Delivery Systems. J. Pharm. Sci. 2020, 109, 3262–3281. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Li, J.; He, S.; Huang, J.; Pu, K. A Renal-Clearable Macromolecular Reporter for Near-Infrared Fluorescence Imaging of Bladder Cancer. Angew. Chemie Int. Ed. 2020, 59, 4415–4420. [Google Scholar] [CrossRef]
- Kumar, K.; Dhawan, N.; Sharma, H.; Vaidya, S.; Vaidya, B. Bioadhesive Polymers: Novel Tool for Drug Delivery. Artif. Cells Nanomedicine Biotechnol. 2014, 42, 274–283. [Google Scholar] [CrossRef]
- Estrellas, K.M.; Fiecas, M.; Azagury, A.; Laulicht, B.; Cho, D.Y.; Mancini, A.; Reineke, J.; Furtado, S.; Mathiowitz, E. Time-Dependent Mucoadhesion of Conjugated Bioadhesive Polymers. Colloids Surf. B Biointerfaces 2019, 173, 454–469. [Google Scholar] [CrossRef]
- Laurén, P.; Paukkonen, H.; Lipiäinen, T.; Dong, Y.; Oksanen, T.; Räikkönen, H.; Ehlers, H.; Laaksonen, P.; Yliperttula, M.; Laaksonen, T. Pectin and Mucin Enhance the Bioadhesion of Drug Loaded Nanofibrillated Cellulose Films. Pharm. Res. 2018, 35. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Pang, X.; Liu, M.; Zhang, B.; Li, L.; Zhai, G. Recent Progresses in Bioadhesive Microspheres via Transmucosal Administration. Colloids Surf. B Biointerfaces 2016, 140, 361–372. [Google Scholar] [CrossRef]
- Bruschi, M.L.; de Francisco, L.M.B.; Borghi, F.B. An Overview of Recent Patents on Composition of Mucoadhesive Drug Delivery Systems. Recent Pat. Drug Deliv. Formul. 2015, 9, 79–87. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M.A. Mucoadhesive Hydrogel Nanoparticles as Smart Biomedical Drug Delivery System. Appl. Sci. 2019, 9, 825. [Google Scholar] [CrossRef]
- Ali, M.S.; Metwally, A.A.; Fahmy, R.H.; Osman, R. Chitosan-Coated Nanodiamonds: Mucoadhesive Platform for Intravesical Delivery of Doxorubicin. Carbohydr. Polym. 2020, 245, 116528. [Google Scholar] [CrossRef]
- Xu, X.; Liu, K.; Jiao, B.; Luo, K.; Ren, J.; Zhang, G.; Yu, Q.; Gan, Z. Mucoadhesive Nanoparticles Based on ROS Activated Gambogic Acid Prodrug for Safe and Efficient Intravesical Instillation Chemotherapy of Bladder Cancer. J. Control. Release 2020, 324, 493–504. [Google Scholar] [CrossRef]
- Lu, S.; Neoh, K.G.; Kang, E.-T.; Mahendran, R.; Chiong, E. Mucoadhesive Polyacrylamide Nanogel as a Potential Hydrophobic Drug Carrier for Intravesical Bladder Cancer Therapy. Eur. J. Pharm. Sci. 2015, 72, 57–68. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan/β-Glycerophosphate in Situ Gelling Mucoadhesive Systems for Intravesical Delivery of Mitomycin-C. Int. J. Pharm. X 2019, 1, 100007. [Google Scholar] [CrossRef]
- Denora, N.; Lopedota, A.; Perrone, M.; Laquintana, V.; Iacobazzi, R.M.; Milella, A.; Fanizza, E.; Depalo, N.; Cutrignelli, A.; Lopalco, A.; et al. Spray-Dried Mucoadhesives for Intravesical Drug Delivery Using N-Acetylcysteine- and Glutathione-Glycol Chitosan Conjugates. Acta Biomater. 2016, 43, 170–184. [Google Scholar] [CrossRef]
- Karavana, S.Y.; Şenyiğit, Z.A.; Çalışkan, Ç.; Sevin, G.; Özdemir, D.İ.; Erzurumlu, Y.; Şen, S.; Baloğlu, E. Gemcitabine Hydrochloride Microspheres Used for Intravesical Treatment of Superficial Bladder Cancer: A Comprehensive in Vitro/Ex Vivo/in Vivo Evaluation. Drug Des. Devel. Ther. 2018, 12, 1959–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Yeh, T.; Wang, J.; Chen, L.; Lyness, G.; Xin, Y.; Wientjes, M.G.; Bergdall, V.; Couto, G.; Alvarez-berger, F.; et al. Paclitaxel Gelatin Nanoparticles for Intravesical Bladder Cancer Therapy. J. Urol. 2011, 185, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xu, L.; Tang, E.; Mahendran, R.; Chiong, E.; Gee, K. Co-Delivery of Peptide-Modified Cisplatin and Doxorubicin via Mucoadhesive Nanocapsules for Potential Synergistic Intravesical Chemotherapy of Non-Muscle-Invasive Bladder Cancer. Eur. J. Pharm. Sci. 2016, 84, 103–115. [Google Scholar] [CrossRef] [PubMed]
- GuhaSarkar, S.; More, P.; Banerjee, R. Urothelium-Adherent, Ion-Triggered Liposome-in-Gel System as a Platform for Intravesical Drug Delivery. J. Control. Release 2017, 245, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.B.; Mahamana, R.; Pal, E. Review on Mucoadhesive Drug Delivery System: Novel Approaches in Modern Era. RGUHS J. Pharm. Sci. 2014, 4, 128–141. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Synthesis and Evaluation of Boronated Chitosan as a Mucoadhesive Polymer for Intravesical Drug Delivery. J. Pharm. Sci. 2019, 108, 3046–3053. [Google Scholar] [CrossRef]
- Muppalaneni, S.; Mastropietro, D.; Omidian, H. Mucoadhesive Drug Delivery Systems. In Engineering Polymer Systems for Improved Drug Delivery; Bader, R.A., Putnam, D.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 9781118747896. [Google Scholar]
- Chatterjee, B.; Amalina, N.; Sengupta, P.; Mandal, U.K. Mucoadhesive Polymers and Their Mode of Action: A Recent Update. J. Appl. Pharm. Sci. 2017. [Google Scholar] [CrossRef]
- Tekade, M.; Maheshwari, N.; Youngren-Ortiz, S.R.; Pandey, V.; Chourasiya, Y.; Soni, V.; Deb, P.K.; Sharma, M.C. Thiolated-Chitosan: A Novel Mucoadhesive Polymer for Better-Targeted Drug Delivery; Elsevier Inc.: London, UK, 2019; ISBN 9780128144282. [Google Scholar]
- Bassi da Silva, J.; de Ferreira, S.B.S.; de Freitas, O.; Bruschi, M.L. A Critical Review about Methodologies for the Analysis of Mucoadhesive Properties of Drug Delivery Systems. Drug Dev. Ind. Pharm. 2017, 43, 1053–1070. [Google Scholar] [CrossRef]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in Mucoadhesive Drug-Delivery Systems: A Brief Note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef]
- Singh, I.; Rana, V. Techniques for the Assessment of Mucoadhesion in Drug Delivery Systems: An Overview. J. Adhes. Sci. Technol. 2012, 26, 2251–2267. [Google Scholar] [CrossRef]
- Roy, S.K.; Prabhakar, B. Bioadhesive Polymeric Platforms for Transmucosal Drug Delivery Systems—A Review. Trop. J. Pharm. Res. 2010, 9, 91–104. [Google Scholar] [CrossRef] [Green Version]
- De Souza Ferreira, S.B.; Moço, T.D.; Borghi-Pangoni, F.B.; Junqueira, M.V.; Bruschi, M.L. Rheological, Mucoadhesive and Textural Properties of Thermoresponsive Polymer Blends for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2016, 55, 164–178. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Ferreira, S.; Reis, A.; Cook, M.; Bruschi, M. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef]
- Huang, D.; Lin, B.; Song, Y.; Guan, Z.; Cheng, J.; Zhu, Z.; Yang, C. Staining Traditional Colloidal Gold Test Strips with Pt. Nanoshell Enables Quantitative Point-of-Care Testing with Simple and Portable Pressure Meter Readout. ACS Appl. Mater. Interfaces 2019, 11, 1800–1806. [Google Scholar] [CrossRef]
- PARK, K. A New Approach to Study Mucoadhesion: Colloidal Gold Staining. Int. J. Pharm. 1989, 53, 209–217. [Google Scholar] [CrossRef]
- Teng, C.L.C.; Ho, N.F.H. Mechanistic Studies in the Simultaneous Flow and Adsorption of Polymer-Coated Latex Particles on Intestinal Mucus I: Methods and Physical Model Development. J. Control. Release 1987, 6, 133–149. [Google Scholar] [CrossRef]
- Efiana, N.A.; Mahmood, A.; Lam, H.T.; Zupančič, O.; Leonaviciute, G.; Bernkop-Schnürch, A. Improved Mucoadhesive Properties of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) by Introducing Acyl Chitosan. Int. J. Pharm. 2017, 519, 206–212. [Google Scholar] [CrossRef]
- Dyawanapelly, S.; Koli, U.; Dharamdasani, V.; Jain, R.; Dandekar, P. Improved Mucoadhesion and Cell Uptake of Chitosan and Chitosan Oligosaccharide Surface-Modified Polymer Nanoparticles for Mucosal Delivery of Proteins. Drug Deliv. Transl. Res. 2016, 6, 365–379. [Google Scholar] [CrossRef]
- Kumari, P.V.K.; Anitha, S.; Rao, Y.S. Review on Pharmacoscintigraphy. J. Pharm. Res. Int. 2020, 32, 23–32. [Google Scholar] [CrossRef]
- Fischer, F.; Bauer, S. Polyvinylpyrrolidon. Ein Tausendsassa in Der Chemie. Chemie unserer Zeit 2009, 43, 376–383. [Google Scholar] [CrossRef]
- Grant, J.J.; Pillai, S.C.; Perova, T.S.; Hehir, S.; Hinder, S.J.; McAfee, M.; Breen, A. Electrospun Fibres of Chitosan/PVP for the Effective Chemotherapeutic Drug Delivery of 5-Fluorouracil. Chemosensors 2021, 9, 70. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Zhang, Z.; Zhang, J.; Huang, M.; Wang, S.; Xie, P. Preparation of Electrospray ALG/PDA–PVP Nanocomposites and Their Application in Cancer Therapy. Soft Matter 2020, 16, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Marušincová, H.; Husárová, L.; Růžička, J.; Ingr, M.; Navrátil, V.; Buňková, L.; Koutny, M. Polyvinyl Alcohol Biodegradation under Denitrifying Conditions. Int. Biodeterior. Biodegradation 2013, 84, 21–28. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl Alcohol Based-Drug Delivery Systems for Cancer Treatment. Int. J. Pharm. 2021, 600. [Google Scholar] [CrossRef]
- Ben Halima, N. Poly(Vinyl Alcohol): Review of Its Promising Applications and Insights into Biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Ullah, K.; Sohail, M.; Murtaza, G.; Khan, S.A. Natural and Synthetic Materials Based CMCh/PVA Hydrogels for Oxaliplatin Delivery: Fabrication, Characterization, In-Vitro and In-Vivo Safety Profiling. Int. J. Biol. Macromol. 2019, 122, 538–548. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- WICHTERLE, O.; LÍM, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Brady, J.; Dürig, t.; Lee, P.I.; Li, J.X. Chapter 7—Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms; Academic Press: Cambridge, MA, USA, 2017; pp. 181–223. ISBN 9780128024478. [Google Scholar]
- Brown, H.P. Carboxylic Polymers 1957. US Patent US2798053A, 2 July 1957. [Google Scholar]
- B F Goodrich Bulletin. Sustained Release Patents Using Carbopol Resin; B F Goodrich Bulletin: Cleveeland, OH, USA, 1987. [Google Scholar]
- Panzade, P.; Puranik, P.K. Carbopol Polymers: A Versatile Polymer for Pharmaceutical Applications. Res. J. Pharm. Technol. 2010, 3, 672–675. [Google Scholar]
- Xu, H.; Wen, Y.; Chen, S.; Zhu, L.; Feng, R.; Song, Z. Paclitaxel Skin Delivery by Micelles-Embedded Carbopol 940 Hydrogel for Local Therapy of Melanoma. Int. J. Pharm. 2020, 587, 119626. [Google Scholar] [CrossRef]
- Harris, J.M. Poly (Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® Block-Copolymers in Medicine: From Chemical and Biological Versatility to Rationalisation and Clinical Advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Ottenbrite, R.M.; Javan, R. Encyclopedia of Condensed Matter Physics; Academic Press: Cambridge, MA, USA, 2005; pp. 99–108. [Google Scholar]
- Şenyiğit, Z.A.; Karavana, S.Y.; İlem-Özdemir, D.I.; Çalışkan, C.; Waldner, C.; Şen, S.; Bernkop-Schnürch, A.; Baloğlu, E. Design and Evaluation of an Intravesical Delivery System for Superficial Bladder Cancer: Preparation of Gemcitabine HCl-Loaded Chitosan-Thioglycolic Acid Nanoparticles and Comparison of Chitosan/Poloxamer Gels as Carriers. Int. J. Nanomedicine 2015, 10, 6493–6507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajazuddin, A.A.; Khan, J.; Giri, T.K.; Tripathi, D.K.; Saraf, S.; Saraf, S. Advancement in Stimuli Triggered in Situ Gelling Delivery for Local and Systemic Route. Expert Opin. Drug Deliv. 2012, 9, 1573–1592. [Google Scholar] [CrossRef] [PubMed]
- Suman, K.; Shanbhag, S.; Joshi, Y.M. Phenomenological Model of Viscoelasticity for Systems Undergoing Sol-Gel Transition. Phys. Fluids 2021, 33. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, W.; Yu, J.; Li, Y.; Zhao, C. Recent Development of PH-Responsive Polymers for Cancer Nanomedicine. Molecules 2018, 24, 4. [Google Scholar] [CrossRef]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and Thermogelling Systems for Vaginal Drug Delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic Gels: Past, Present and Future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnürch, A. In Situ Gelling and Mucoadhesive Polymers: Why Do They Need Each Other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef]
- Lihong, W.; Xin, C.; Yongxue, G.; Yiying, B.; Gang, C. Thermoresponsive Ophthalmic Poloxamer/Tween/Carbopol in Situ Gels of a Poorly Water-Soluble Drug Fluconazole: Preparation and in Vitro – in Vivo Evaluation. Drug Dev. Ind. Pharm. 2014, 40, 1402–1410. [Google Scholar] [CrossRef]
- Matanović, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive Polymers: Insights into Decisive Hydrogel Characteristics, Mechanisms of Gelation, and Promising Biomedical Applications. Int. J. Pharm. 2014, 472, 262–275. [Google Scholar] [CrossRef]
- Park, H.; Kim, M.H.; Yoon, Y.I.; Park, W.H. One-Pot Synthesis of Injectable Methylcellulose Hydrogel Containing Calcium Phosphate Nanoparticles. Carbohydr. Polym. 2017, 157, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N. Thermal Gelation Properties of Methyl and Hydroxypropyl Methylcellulose. J. Appl. Polym. Sci. 1979, 24, 1073–1087. [Google Scholar] [CrossRef]
- Sharma, M.; Deohra, A.; Reddy, K.R.; Sadhu, V. Biocompatible In-Situ Gelling Polymer Hydrogels for Treating Ocular Infection. In Methods in Microbiology; Academic Press: Cambridge, MA, USA, 2019; pp. 93–114. [Google Scholar]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From Controlled Release to PH-Responsive Drug Delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Gupta, S. Carbopol/Chitosan Based PH Triggered In Situ Gelling System for Ocular Delivery of Timolol Maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent Advances in the Development of In Situ Gelling Drug Delivery Systems for Non-Parenteral Administration Routes. Pharmaceutics 2020, 12, 859. [Google Scholar] [CrossRef]
- CAO, S.; REN, X.; ZHANG, Q.; CHEN, E.; XU, F.; CHEN, J.; LIU, L.; JIANG, X. In Situ Gel Based on Gellan Gum as New Carrier for Nasal Administration of Mometasone Furoate. Int. J. Pharm. 2009, 365, 109–115. [Google Scholar] [CrossRef]
- Mi, F.-L.; Shyu, S.-S.; Peng, C.-K. Characterization of Ring-Opening Polymerization of Genipin and PH-Dependent Cross-Linking Reactions between Chitosan and Genipin. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1985–2000. [Google Scholar] [CrossRef]
- Narita, T.; Yunoki, S.; Ohyabu, Y.; Yahagi, N.; Uraoka, T. In Situ Gelation Properties of a Collagen–Genipin Sol with a Potential for the Treatment of Gastrointestinal Ulcers. Med. Devices Evid. Res. 2016, 9, 429–439. [Google Scholar] [CrossRef]
- Simões, A.; Miranda, M.; Cardoso, C.; Vitorino, F. Rheology by Design: A Regulatory Tutorial for Analytical Method Validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef]
- Qwist, P.K.; Sander, C.; Okkels, F.; Jessen, V.; Baldursdottir, S.; Rantanen, J. On-Line Rheological Characterization of Semi-Solid Formulations. Eur. J. Pharm. Sci. 2019, 128, 36–42. [Google Scholar] [CrossRef]
- Ghica, M.V.; Hîrjău, M.; Lupuleasa, D.; Dinu-Pîrvu, C.-E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Mol. 2016, Vol. 21, Page 786 2016, 21, 786. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Choudhury, A.R. Synthesis and Rheological Studies of a Novel Composite Hydrogel of Xanthan, Gellan and Pullulan. Int. J. Biol. Macromol. 2019, 137, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.R. PH Mediated Rheological Modulation of Chitosan Hydrogels. Int. J. Biol. Macromol. 2020, 156, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Naik, P.K.; Pradhan, D.; Ghosh, G.; Rath, G. Mucoadhesive Formulations: Innovations, Merits, Drawbacks, and Future Outlook. Pharm. Dev. Technol. 2020, 25, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.W.; Yoon, H.Y.; Yang, H.M.; Kim, C.H.; Goo, Y.T.; Kang, M.J.; Lee, S. Current Status of the Development of Intravesical Drug Delivery Systems for the Treatment of Bladder Cancer. Expert Opin. Drug Deliv. 2020, 17, 1555–1572. [Google Scholar] [CrossRef]










| Drug | Carrier | Polymer | Cancer Cells | Encapsulation Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Doxorubicin | Nanodiamonds with surface modification | Chitosan | HT-1197 | >90 | [37] |
| Doxorubicin | Nanoparticles with surface modification | Poly(amidoamine) | UMUC3 | >90 | [27] |
| Gambogic acid | Nanoparticles with surface modification | Chitosan | MB49 and MH-3T3 | - | [38] |
| Docetaxel | Nanogel | Polyacrylamide | UMC3 and T24 | >90 | [39] |
| MMC | Gel | Chitosan/β-glycerophosphate | - | - | [40] |
| Fluorescein diacetate | Micro and nanoparticles | CH glycol (GCH), N-acetylcysteine (NAC), and glutadione (GSH) | - | 12.2–100% | [41] |
| Gemcitabine hydrochloride | Microspheres | Carbopol 2020 NF, Eudragit E100 (EE100), poloxamer and chitosan | T24 (ATCC HTB4TM) and RT4 (ATCC HTB2TM) | >80 | [42] |
| Paclitaxel | Nanoparticles | Gelatin | - | 0.52 | [43] |
| Doxorubicin and peptide-modified cisplatin | Nanocapsules | Chitosan, polymethacrylic acid | UMUC3 | >80 | [44] |
| Paclitaxel | Liposomes in a gel system | Gellan gum | NBT-II and T24 (ATCC, USA) | >90 | [45] |
| Advantages | Disadvantages |
|---|---|
| Prolong drug residence time at the tumor site | Dislodgement of the formulation may happen |
| Increase drug bioavailability | Overhydration may compromise the formulation structure |
| Reduce dosing frequency | |
| Improve drug permeability | |
| Reduce the dose of drug administered | |
| Fast onset of action |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima, C.S.A.; Varca, J.P.R.O.; Alves, V.M.; Nogueira, K.M.; Cruz, C.P.C.; Rial-Hermida, M.I.; Kadłubowski, S.S.; Varca, G.H.C.; Lugão, A.B. Mucoadhesive Polymers and Their Applications in Drug Delivery Systems for the Treatment of Bladder Cancer. Gels 2022, 8, 587. https://doi.org/10.3390/gels8090587
de Lima CSA, Varca JPRO, Alves VM, Nogueira KM, Cruz CPC, Rial-Hermida MI, Kadłubowski SS, Varca GHC, Lugão AB. Mucoadhesive Polymers and Their Applications in Drug Delivery Systems for the Treatment of Bladder Cancer. Gels. 2022; 8(9):587. https://doi.org/10.3390/gels8090587
Chicago/Turabian Stylede Lima, Caroline S. A., Justine P. R. O. Varca, Victória M. Alves, Kamila M. Nogueira, Cassia P. C. Cruz, M. Isabel Rial-Hermida, Sławomir S. Kadłubowski, Gustavo H. C. Varca, and Ademar B. Lugão. 2022. "Mucoadhesive Polymers and Their Applications in Drug Delivery Systems for the Treatment of Bladder Cancer" Gels 8, no. 9: 587. https://doi.org/10.3390/gels8090587