Macroalgal-Derived Alginate Soil Amendments for Water Retention, Nutrient Release Rate Reduction, and Soil pH Control
Abstract
:1. Introduction
2. Results and Discussion
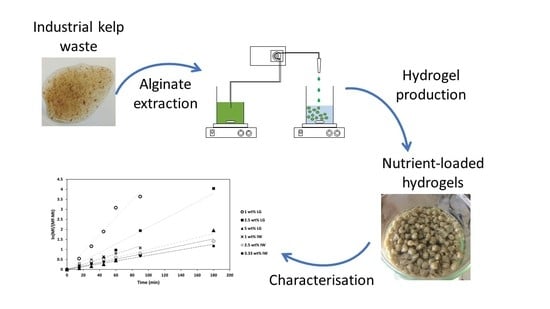
2.1. Production of Alginate-Based Hydrogels
2.2. Guluronic Acid to Mannuronic Acid Ratio
2.3. Water Retention Tests with Produced Hydrogels
2.4. Alginate Based Slow-Release Fertilisers
2.4.1. Nutrient Concentrations over Time
2.4.2. Diffusivity of the Nutrients from Produced Slow-Release Fertilisers
2.5. Using Encapsulated Limestone for pH Control
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Experimental Methods
4.2.1. Solid Content of Industrial Kelp Solid Waste
4.2.2. Alginate Content of Industrial Kelp Solid Waste
4.2.3. Soluble Alginate Produced from Kelp Biomass
4.2.4. Guluronic to Mannuronic Acid Ratio in Alginate
4.2.5. Production of Alginate-Based Hydrogel Beads
4.2.6. Water Uptake and Retention Tests
4.2.7. Production of LG Alginate-Based Slow-Release Fertilisers
4.2.8. Production of Kelp-Derived Alginate-Based Slow-Release Fertilisers
4.2.9. Production of Limestone Loaded Hydrogels
4.2.10. Nutrient Release Rates of Slow-Release Fertilisers
4.2.11. Diffusivity Calculations
4.2.12. Solution pH Analysis with the Addition of Produced Hydrogel Beads
4.3. Analytical Methods
4.3.1. Phenol-Sulfuric Acid Method
4.3.2. Atomic Absorption Spectroscopy Analysis
4.3.3. Spectrophotometry Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huho, J.M.; Ngaira, J.K.W.; Ogindo, H.O.; Masayi, N. The changing rainfall pattern and the associated impacts on subsistence agriculture in Laikipia East District, Kenya. J. Geogr. Reg. Plan. 2012, 5, 198–206. [Google Scholar] [CrossRef]
- Du Plessis, J.A.; Burger, G.J. Investigation into increasing short-duration rainfall intensities in South Africa. Water SA 2015, 41, 416–424. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Bogard, M.J.; Donald, D.B.; Finlay, K.; Leavitt, P.R. Distribution and regulation of urea in lakes of central North America. Freshwat. Biol. 2012, 57, 1277–1292. [Google Scholar] [CrossRef]
- Zafari, A.; Kianmehr, M.H. Management and reduction of chemical nitrogen consumption in agriculture. Am. J. Plant Sci. 2012, 3, 1827–1834. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S.; Xie, L.; Wang, Y. Environmentally friendly slow-release nitrogen fertilizer. J. Agric. Food Chem. 2011, 59, 10169–10175. [Google Scholar] [CrossRef] [PubMed]
- Rashidzadeh, A.; Olad, A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly(AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr. Polym. 2014, 114, 269–278. [Google Scholar] [CrossRef]
- Orikiriza, L.J.B.; Agaba, H.; Tweheyo, M.; Eilu, G.; Kabasa, J.D.; Hüttermann, A. Amending soils with hydrogels increases the biomass of nine tree species under non-water stress conditions. Clean Soil Air Water 2009, 37, 615–620. [Google Scholar] [CrossRef]
- Davidson, D.W.; Verma, M.S.; Gu, F.X. Controlled root targeted delivery of fertilizer using an ionically crosslinked carboxymethyl cellulose hydrogel matrix. SpringerPlus 2013, 2, 318. [Google Scholar] [CrossRef]
- Oshunsanya, S.O. Introductory Chapter: Relevance of Soil pH to Agriculture. In Soil pH for Nutrient Availability and Crop Performance; Oshunsanya, S.O., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Schwaeble, C.F.; Pott, R.W.M.; Goosen, N.J. The effect of sodium alginate, lignosulfonate and bentonite binders on agglomeration performance and mechanical strength of micro-fine agricultural lime pellets. Part. Sci. Technol. 2021, 39, 820–831. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xu, L.; Liu, Y.; Qui, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. Applications of absorbent polymers for sustainable plant protection and crop yield. Sustainability 2021, 13, 3253. [Google Scholar] [CrossRef]
- Seetapan, N.; Wongsawaeng, J.; Kiatkamjornwong, S. Gel strength and swelling of acrylamide-protic acid superabsorbent copolymers. Polym. Adv. Technol. 2011, 22, 1685–1695. [Google Scholar] [CrossRef]
- Sharma, R.; Bajpai, J.; Bajpai, A.K.; Acharya, S.; Shrivastava, R.B.; Shukla, S.K. Designing slow water-releasing alginate nanoreserviors for sustained irrigation in scanty rainfall areas. Carbohydr. Polym. 2014, 102, 513–520. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Fereres, E.; Mata, M.; Girona, J.; Cohen, M. Sensitivity of Continuous and Discrete Plant and Soil Water Status Monitoring in Peach Trees Subjected to Deficit Irrigation. J. Am. Soc. Hortic. Sci. 1999, 124, 437–444. [Google Scholar] [CrossRef]
- Flanagan, D.C.; Chaudhari, K.; Norton, L.D. Polyacrylamide soil amendment effects on runoff and sediment yield on steep slopes: PART II. natural rainfall conditions. Trans. Am. Soc. Agric. Eng. 2002, 45, 1339–1351. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2020, 138, 50376. [Google Scholar] [CrossRef]
- Sofyane, A.; Ablouh, E.; Lahcini, M.; Elmeziane, A.; Khouloud, M.; Kaddami, H.; Raihane, M. Slow-release fertilizers based on starch acetate/glycerol/polyvinyl alcohol biocomposites for sustained nutrient release. Mater. Today Proc. 2020, 36, 74–81. [Google Scholar] [CrossRef]
- An, D.; Yang, L.; Liu, B.; Wang, T.J.; Kan, C. Diffusion performance of fertilizer nutrient through polymer latex film. J. Agric. Food Chem. 2017, 65, 10868–10874. [Google Scholar] [CrossRef] [PubMed]
- Achmon, Y.; Dowdy, F.R.; Simmons, C.W.; Zohar-Perez, C.; Rabinovitz, Z.; Nussinovitch, A. Degradation and bioavailability of dried alginate hydrocolloid capsules in simulated soil system. J. Appl. Polym. Sci. 2019, 136, 48142. [Google Scholar] [CrossRef]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement strategies for advanced applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Imeson, A. Thickening and Gelling Agents for Food; Blackie: London, UK, 1992. [Google Scholar]
- Gao, J.; Li, Z.; Wang, Z.; Chen, T.; Hu, G.; Zhao, Y.; Han, X. Facile synthesis of sustainable tannin/sodium alginate composite hydrogel beads for efficient removal of methylene blue. Gels 2022, 8, 486. [Google Scholar] [CrossRef]
- Zactiti, E.M.; Kieckbusch, T.G. Release of potassium sorbate from active films of sodium alginate crosslinked with calcium chloride. Packag. Technol. Sci. 2009, 22, 349–358. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Bianco-Peled, H. A quantitative analysis of alginate swelling. Carbohydr. Polym. 2010, 79, 1020–1027. [Google Scholar] [CrossRef]
- Gallagher, L.; Smith, A.; Kavanagh, K.; Devereux, M.; Colleran, J.; Breslin, C.; Richards, K.; McCann, M.; Rooney, A.D. Preparation and antimicrobial properties of alginate and serum albumin/glutaraldehyde hydrogels impregnated with silver(I) ions. Chemistry 2021, 3, 672–686. [Google Scholar] [CrossRef]
- Abd El-Rehim, H.A. Characterization and possible agricultural application of polyacrylamide/sodium alginate crosslinked hydrogels prepared by ionizing radiation. J. Appl. Polym. Sci. 2006, 101, 3572–3580. [Google Scholar] [CrossRef]
- Shivakumara, L.R.; Demappa, T. Synthesis and swelling behavior of sodium alginate/poly(Vinyl alcohol) hydrogels. Turk. J. Pharm. Sci. 2019, 16, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Gopinath, K.P.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- Mazen, A.M.; Radwan, D.E.M.; Ahmed, A.F. Growth responses of maize plants cultivated in sandy soil amended by different superabsorbant hydrogels. J. Plant Nutr. 2015, 38, 325–337. [Google Scholar] [CrossRef]
- Idrissi, A.E.; Gharrak, A.E.; Achagri, G.; Essamlali, Y.; Amadine, O.; Akil, A.; Sair, S.; Zahouil, M. Synthesis of urea-containing sodium alginate-g-poly(acrylic acid-co-acrylamide) superabsorbent-fertilizer hydrogel reinforced with carboxylated cellulose nanocrystals for efficient water and nitrogen utilization. J. Environ. Chem. Eng. 2022, 10, 108282. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal compost—Toward sustainable fertilization. Rev. Inorg. Chem. 2013, 33, 161–172. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, R.J.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Applications of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef]
- Lötze, E.; Hoffman, E.W. Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 2016, 28, 1379–1386. [Google Scholar] [CrossRef]
- Fatehi, H.; Ong, D.E.L.; Yu, J.; Chang, I. Biopolymers as green binders for soil improvement in geotechnical applications: A review. Geosciences 2021, 11, 291. [Google Scholar] [CrossRef]
- Pérez-Hernández, H.; Fernández-Luqueño, F.; Huerta-Lwanga, E.; Mendoza-Vega, J.; José, D.Á.-S. Effect of engineered nanoparticles on soil biota: Do they improve the soil quality and crop production or jeopardize them? Land Degrad. Dev. 2020, 31, 2213–2230. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Theodoropoulos, A.G.; Drossopoulos, J.B. 1995. Designing synthetic polymers as soil conditioners. Commun. Soil Sci. Plant Anal. 1995, 26, 1455–1480. [Google Scholar] [CrossRef]
- Hüttermann, A.; Zommorodi, M.; Reise, K. Addition of hydrogels to soil for prolonging the survival of Pinus halepensis seedlings subjected to drought. Soil Tillage Res. 1999, 50, 295–304. [Google Scholar] [CrossRef]
- Campos, E.V.R.; De Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2014, 35, 47–66. [Google Scholar] [CrossRef]
- Lackey, B.A.; Muldoon, A.E.; Jaffee, B.A. Alginate pellet formulation of hirsutella rhossiliensis for biological control of plant-parasitic nematodes. Biol. Control 1993, 3, 155–160. [Google Scholar] [CrossRef]
- Ekanayake, S.A.; Godakumbura, P.I. Synthesis of a dual-functional nanofertilizer by embedding ZnO and CuO nanoparticles on an alginate-based hydrogel. ACS Omega 2021, 6, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Knijnenburg, J.T.N.; Kasemsiri, P.; Amornrantanaworn, K.; Suwanree, S.; Iamamornphan, W.; Chindaprasirt, P.; Jetsrisuparb, K. Entrapment of nano-ZnO into alginate/polyvinyl alcohol beads with different crosslinking ions for fertilizer applications. Int. J. Biol. Macromol. 2021, 181, 349–356. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Q.; Sun, X.; Yue, Y.; Tubana, B.; Yang, R.; Cheng, H.N. Novel alginate-cellulose nanofiber-poly(vinyl alcohol) hydrogels for carrying and delivering nitrogen, phosphorus and potassium chemicals. Int. J. Biol. Macromol. 2021, 172, 330–340. [Google Scholar] [CrossRef]
- Di Martino, A.; Khan, Y.A.; Durpekova, S.; Sedlarik, V.; Elich, O.; Cechmankova, J. Ecofriendly renewable hydrogels based on whey protein and for slow release of fertilizers and soil conditioning. J. Clean. Prod. 2021, 285, 1248484. [Google Scholar] [CrossRef]
- Arafa, E.G.; Sabaa, M.W.; Mohamed, R.R.; Elzanaty, A.M.; Abdel-Gawad, O.F. Preparation of biodegradable sodium alginate/carboxymethylchitosan hydrogels for the slow-release of urea fertilizer and their antimicrobial activity. React. Funct. Polym. 2022, 174, 105243. [Google Scholar] [CrossRef]
- Van der Merwe, R.D.T. Macroalgal-Derived Alginate Soil Amendments for Water Retention, Reduced Nutrient Release Rate, and Soil pH Control; Stellenbosch University: Stellenbosch, South Africa, 2022. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry; Food and Agricultural Organisation of the United Nations: Rome, Italy, 2003; ISBN 92-5-104958-0. [Google Scholar]
- Zhao, Y.; Chen, H.H.; Wang, Y.S.; Li, Q.Q. Effect of sodium alginate and its guluronic acid/mannuronic acid ratio on the physicochemical properties of high-amylose cornstarch. Starch Staerke 2016, 68, 1215–1223. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of alginate-based biomaterials and their applications in biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Skjåk-Bræk, G.; Draget, K.I. Alginates: Properties and Applications. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Devi, G.K.; Kumar, P.S.; Kumar, K.S. Green synthesis of novel silver nanocomposite hydrogel based on sodium alginate as an efficient biosorbent for the dye wastewater treatment: Prediction of isotherm and kinetic parameters. Desalination Water Treat. 2016, 57, 27686–27699. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Ruiz-Pividal, J.F.; Llorens-Gámez, M. Enhancement of water diffusion and compression performance of crosslinked alginate films with a minuscule amount of graphene oxide. Sci. Rep. 2017, 7, 11684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Feng, W.; Jin, C. Protocol efficiently measuring the swelling rate of hydrogels. MethodsX 2020, 7, 100779. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, D. A comprehensive analysis of alginate content and biochemical composition of leftover pulp from brown seaweed Sargassum wightii. Algal Res. 2017, 23, 233–239. [Google Scholar] [CrossRef]
- Llive, L.M.; Perullini, M.; Santagapita, P.R.; Schneider-teixeira, A.; Deladino, L. Controlled release of fertilizers from Ca (II)-alginate matrix modified by yerba mate (Ilex paraguariensis) waste. Eur. Polym. J. 2020, 138, 109955. [Google Scholar] [CrossRef]
- Mandal, S.; Kumar, S.S.; Krishnamoorthy, B.; Basu, S.K. Development and evaluation of calcium alginate beads prepared by sequential and simultaneous methods. Braz. J. Pharm. Sci. 2010, 46, 785–793. [Google Scholar] [CrossRef]
- Lide, D.R.; Baysinger, G.; Berger, L.I.; Goldberg, R.N.; Kehiaian, H.V.; Kuchitsu, K.; Rosenblatt, G.; Roth, D.L.; Zwillinger, D. CRC Handbook of Chemistry and Physics, 88th ed.; CRC Press: London, UK, 2007. [Google Scholar]
- Papancea, A.; Patachia, S. Electrolytes diffusion through modified PVA hydrogel membrane. Bull. Transilv. Univ. Bras. 2014, 7, 53–60. [Google Scholar]
- Fageria, N.K.; Nascente, A.S. Chapter Six—Management of Soil Acidity of South American Soils for Sustainable Crop Production. In Advances in Agronomy; Sparks, D.S., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 128, pp. 221–275. [Google Scholar]
- Schwab, G.; Murdock, L.W.; Ditsch, D.; Rasnake, M.; Sikora, F.J.; Frye, W. Agricultural Lime Recommendations Based on Lime Quality; University of Kentucky—Cooperative Extension Service, College of Agriculture: Lexington, KY, USA, 2007; pp. 1–6. [Google Scholar]
- Gomez, C.G.; Lambrecht, M.V.P.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction-purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.A.; Kang, K.H.; Kim, S.K. Seaweed polysaccharides and their potential biomedical applications. Starch/Staerke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples—NREL/TP-510-42623; National Renewable Energy Laboratory: Golden, CO, USA, 2008; pp. 1–14. [Google Scholar]
- Wang, W.; Chen, F.; Wang, Y.; Wang, L.; Fu, H.; Zheng, F.; Beecher, L. Optimization of reactions between reducing sugars and 1-phenyl-3-methyl-5-pyrazolone (PMP) by response surface methodology. Food Chem. 2018, 254, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Rashidzadeh, A.; Olad, A.; Reyhanitabar, A. Hydrogel/clinoptilolite nanocomposite-coated fertilizer: Swelling, water-retention and slow-release fertilizer properties. Polym. Bull. 2015, 72, 2667–2684. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, Y.H. Thermo and pH-responsive methylcellulose and hydroxypropyl hydrogels containing K2SO4 for water retention and a controlled-release water-soluble fertilizer. Sci. Total Environ. 2019, 655, 958–967. [Google Scholar] [CrossRef]
- Lejcuś, K.; Śpitalniak, M.; Dabrowska, J. Swelling behaviour of superabsorbent polymers for soil amendment under different loads. Polymers 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Mostinsky, I.L. Diffusion Coefficient. In A-to-Z Guide to Thermodynamics, Heat and Mass Transfer, and Fluids Engineering; Begellhouse: Danbury, CT, USA, 2008. [Google Scholar] [CrossRef]
- Daintith, J. A Dictionary of Chemistry, 6th ed.; Daintith, J., Ed.; Oxford University Press: Oxford, UK, 2008; p. 224. [Google Scholar]
- Grunwald, P. Determination of effective diffusion coefficients—An important parameters for the efficiency of immobilized biocatalysts. Biochemical Education. Biochem. Educ. 1989, 17, 99–102. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]






| Alginate Source | G/M Ratio | Reference |
|---|---|---|
| IW | 0.36 | This study |
| LG | 0.53 | This study |
| Ecklonia maxima | 0.82 | [56] |
| Macrocystis pyrifera | 0.64 | [56] |
| Alginate Source | (wt%) | r (s) | Se (g/g) | R2 |
|---|---|---|---|---|
| Laboratory-grade (LG) | 1 | 1542 | 0.62 | 0.972 |
| 2.5 | 4326 | 0.71 | 0.974 | |
| 5 | 7797 | 0.85 | 0.983 | |
| Industrial kelp solid waste (IW) | 1 | 4047 | 1.67 | 0.972 |
| 2.5 | 5913 | 1.20 | 0.985 | |
| 3.33 | 7654 | 1.31 | 0.991 |
| Fertiliser Nutrient | Alginate Source | Diffusion Coefficient (cm2/s) | Reference |
|---|---|---|---|
| Potassium ions | LG | 5.02 × 10−6 | This study |
| IW | 4.14 × 10−6 | This study | |
| K+ (in infinite water) | 1.96 × 10−5 | [63] | |
| PVA membrane | 3.91 × 10−6 | [64] | |
| Polymer latex | 1.86 × 10−9 | [22] | |
| Nitrate ions | LG | 7.07 × 10−6 | This study |
| IW | 5.61 × 10−6 | This study | |
| NO3− (in infinite water) | 1.90 × 10−5 | [63] | |
| Polymer latex | 1.25 × 10−9 | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Merwe, R.d.T.; Goosen, N.J.; Pott, R.W.M. Macroalgal-Derived Alginate Soil Amendments for Water Retention, Nutrient Release Rate Reduction, and Soil pH Control. Gels 2022, 8, 548. https://doi.org/10.3390/gels8090548
van der Merwe RdT, Goosen NJ, Pott RWM. Macroalgal-Derived Alginate Soil Amendments for Water Retention, Nutrient Release Rate Reduction, and Soil pH Control. Gels. 2022; 8(9):548. https://doi.org/10.3390/gels8090548
Chicago/Turabian Stylevan der Merwe, Roelof du Toit, Neill Jurgens Goosen, and Robert William McClelland Pott. 2022. "Macroalgal-Derived Alginate Soil Amendments for Water Retention, Nutrient Release Rate Reduction, and Soil pH Control" Gels 8, no. 9: 548. https://doi.org/10.3390/gels8090548