Photomotion of Hydrogels with Covalently Attached Azo Dye Moieties—Thermoresponsive and Non-Thermoresponsive Gels
Abstract
:1. Introduction
2. Results and Discussion
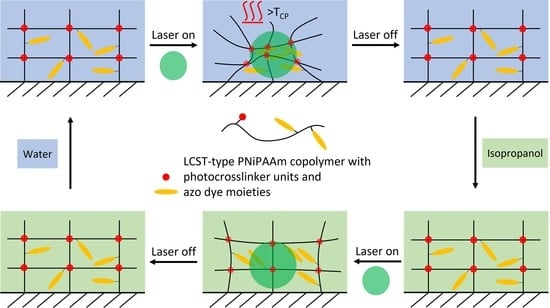
- The ability for photoactuation was compared between a gel of the non-thermoresponsive PNR and a gel of the thermoresponsive P22°C. The LCST-behaviour of P22°C was then turned off by changing the liquid medium to isopropanol, to study the effect of thermoresponsiveness on photoactuation.
- Hydrogels of copolymers with different cloud points were photoactuated at high laser powers (in the range of 3.75 mW) to further elucidate photothermal effects.
- A gel of P22°C was illuminated at different laser powers (from 85 µW to 3.75 mW) to correlate the degree of photoactuation with the light intensity.
2.1. Effect of Thermoresponsiveness
2.2. Cloud-Point Temperature Dependence
2.3. Power Dependence
2.4. Reversibility of Photomotion:
2.5. Photoactuation Models:
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Yang, J.; Zhang, X.; Feng, Y.; Zeng, H.; Wang, L.; Feng, W. Light-driven bimorph soft actuators: Design, fabrication, and properties. Mater. Horiz. 2021, 8, 728–757. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Z.; Wei, Y.-Y.; Wang, W.; Ju, X.-J.; Xie, R.; Chu, L.-Y. Near-Infrared Light-Responsive Poly(N-isopropylacrylamide)/Graphene Oxide Nanocomposite Hydrogels with Ultrahigh Tensibility. ACS Appl. Mater. Interfaces 2015, 7, 27289–27298. [Google Scholar] [CrossRef]
- He, Y.; Liang, H.; Chen, M.; Jiang, L.; Zhang, Z.; Heng, X.; Yang, L.; Hao, Y.; Gan, J.; Yang, Z. Optical Fiber Waveguiding Soft Photoactuators Exhibiting Giant Reversible Shape Change. Adv. Optical Mater. 2021, 9, 2101132. [Google Scholar] [CrossRef]
- Luo, P.-F.; Xiang, S.-L.; Li, C.; Zhu, M.-Q. Photomechanical polymer hydrogels based on molecular photoswitches. J. Polym. Sci. 2021, 59, 2246–2264. [Google Scholar] [CrossRef]
- Peng, X.; Wang, H. Shape changing hydrogels and their applications as soft actuators. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1314–1324. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Quan, M.; Zhang, L.; Yang, H.; Lu, Y. Photothermal effect of azopyridine compounds and their applications. RSC Adv 2015, 5, 4675–4680. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Li, L.; Liu, E.; Zong, C.; Zhao, J.; Xie, J.; Xu, F.; König, T.A.F.; Grenzer Saphiannikova, M.; et al. All-Optical Reversible Azo-Based Wrinkling Patterns with High Aspect Ratio and Polarization-Independent Orientation for Light-Responsive Soft Photonics. ACS Appl. Mater. Interfaces 2019, 11, 25595–25604. [Google Scholar] [CrossRef]
- Lee, K.M.; White, T.J. Photochemical Mechanism and Photothermal Considerations in the Mechanical Response of Monodomain, Azobenzene-Functionalized Liquid Crystal Polymer Networks. Macromolecules 2012, 45, 7163–7170. [Google Scholar] [CrossRef]
- Gelebart, A.H.; Vantomme, G.; Meijer, E.W.; Broer, D.J. Mastering the Photothermal Effect in Liquid Crystal Networks: A General Approach for Self-Sustained Mechanical Oscillators. Adv. Mater. Weinheim. 2017, 29, 1606712. [Google Scholar] [CrossRef]
- Hogan, P.M.; Tajbakhsh, A.R.; Terentjev, E.M. UV manipulation of order and macroscopic shape in nematic elastomers. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002, 65, 41720. [Google Scholar] [CrossRef]
- Homma, K.; Chang, A.C.; Yamamoto, S.; Tamate, R.; Ueki, T.; Nakanishi, J. Design of azobenzene-bearing hydrogel with photoswitchable mechanics driven by photo-induced phase transition for in vitro disease modeling. Acta Biomater. 2021, 132, 103–113. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhang, X.; Zhang, R.; Hu, Y.; Boyer, C.; Xu, F.-J. Photo-responsive supramolecular hyaluronic acid hydrogels for accelerated wound healing. J. Control. Release 2020, 323, 24–35. [Google Scholar] [CrossRef]
- Kuwabara, T.; Aoyagi, T.; Takamura, M.; Matsushita, A.; Nakamura, A.; Ueno, A. Heterodimerization of Dye-Modified Cyclodextrins with Native Cyclodextrins. J. Org. Chem. 2002, 67, 720–725. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Maatz, G.; Maciollek, A.; Ritter, H. Cyclodextrin-induced host-guest effects of classically prepared poly(NIPAM) bearing azo-dye end groups. Beilstein J. Org. Chem. 2012, 8, 1929–1935. [Google Scholar] [CrossRef]
- Guan, Y.; Zhao, H.-B.; Yu, L.-X.; Chen, S.-C.; Wang, Y.-Z. Multi-stimuli sensitive supramolecular hydrogel formed by host–guest interaction between PNIPAM-Azo and cyclodextrin dimers. RSC Adv. 2014, 4, 4955. [Google Scholar] [CrossRef]
- Takashima, Y.; Nakayama, T.; Miyauchi, M.; Kawaguchi, Y.; Yamaguchi, H.; Harada, A. Complex Formation and Gelation between Copolymers Containing Pendant Azobenzene Groups and Cyclodextrin Polymers. Chem. Lett. 2004, 33, 890–891. [Google Scholar] [CrossRef]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion-contraction of photoresponsive artificial muscle regulated by host-guest interactions. Nat. Commun. 2012, 3, 1270. [Google Scholar] [CrossRef]
- Iwaso, K.; Takashima, Y.; Harada, A. Fast response dry-type artificial molecular muscles with c2daisy chains. Nat. Chem. 2016, 8, 625–632. [Google Scholar] [CrossRef]
- Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Photoswitchable supramolecular hydrogels formed by cyclodextrins and azobenzene polymers. Angew. Chem. Int. Ed Engl. 2010, 49, 7461–7464. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kobayashi, Y.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Harada, A. Photoswitchable gel assembly based on molecular recognition. Nat. Commun. 2012, 3, 603. [Google Scholar] [CrossRef]
- Tomatsu, I.; Hashidzume, A.; Harada, A. Cyclodextrin-Based Side-Chain Polyrotaxane with Unidirectional Inclusion in Aqueous Media. Angew. Chem. 2006, 118, 4721–4724. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Yi, S.; Chen, X. Visible-light/temperature dual-responsive hydrogel constructed by α-cyclodextrin and an azobenzene linked surfactant. Soft Matter 2017, 13, 6490–6498. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Li, L.; Wang, J.; Wang, J.; Ma, J.; Yuan, Z.; Lincoln, S.F.; Guo, X. Photo-Reversible Supramolecular Hydrogels Assembled by α-Cyclodextrin and Azobenzene Substituted Poly(acrylic acid)s: Effect of Substitution Degree, Concentration, and Tethered Chain Length. Macromol. Mater. Eng. 2016, 301, 191–198. [Google Scholar] [CrossRef]
- Yuan, W.; Shen, J.; Guo, W. Thermoresponse and light-induced reversible self-assembly/disassembly of supra-amphiphiles from azobenzene- and β-cyclodextrin-containing copolymers. Mater. Lett. 2014, 134, 259–262. [Google Scholar] [CrossRef]
- Yang, M.; Hu, J.; Meng, J.; Shan, X. A thermo and photoresponsive dual performing hydrogel for multiple controlled release mechanisms. Iran Polym. J. 2020, 29, 891–900. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Auer, S.K.; Fossati, S.; Morozov, Y.; Mor, D.C.; Jonas, U.; Dostalek, J. Rapid Actuation of Thermo-Responsive Polymer Networks: Investigation of the Transition Kinetics. J. Phys. Chem. B 2022, 126, 3170–3179. [Google Scholar] [CrossRef]
- Da Pilz Cunha, M.; van Thoor, E.A.J.; Debije, M.G.; Broer, D.J.; Schenning, A.P.H.J. Unravelling the photothermal and photomechanical contributions to actuation of azobenzene-doped liquid crystal polymers in air and water. J. Mater. Chem. C 2019, 7, 13502–13509. [Google Scholar] [CrossRef]
- Gelebart, A.H.; Jan Mulder, D.; Varga, M.; Konya, A.; Vantomme, G.; Meijer, E.W.; Selinger, R.L.B.; Broer, D.J. Making waves in a photoactive polymer film. Nature 2017, 546, 632–636. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Loebner, S.; Yadav, B.; Lomadze, N.; Tverdokhleb, N.; Donner, H.; Saphiannikova, M.; Santer, S. Local Direction of Optomechanical Stress in Azobenzene Containing Polymers During Surface Relief Grating Formation. Macromol. Mater. Eng. 2022, 2100990. [Google Scholar] [CrossRef]
- Yadav, B.; Domurath, J.; Kim, K.; Lee, S.; Saphiannikova, M. Orientation Approach to Directional Photodeformations in Glassy Side-Chain Azopolymers. J. Phys. Chem. B 2019, 123, 3337–3347. [Google Scholar] [CrossRef]
- Yadav, B.; Domurath, J.; Saphiannikova, M. Modeling of Stripe Patterns in Photosensitive Azopolymers. Polymers 2020, 12, 735. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.S.; Park, J.-K. Directional photofluidization lithography: Micro/nanostructural evolution by photofluidic motions of azobenzene materials. Adv. Mater. Weinheim. 2012, 24, 2069–2103. [Google Scholar] [CrossRef]
- Viswanathan, N.K.; Kim, D.Y.; Bian, S.; Williams, J.; Liu, W.; Li, L.; Samuelson, L.; Kumar, J.; Tripathy, S.K. Surface relief structures on azo polymer films. J. Mater. Chem. 1999, 9, 1941–1955. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced Motions in Azo-Containing Polymers. Chem. Rev. 2002, 102, 4139–4176. [Google Scholar] [CrossRef]
- Kumar, J.; Li, L.; Jiang, X.L.; Kim, D.-Y.; Lee, T.S.; Tripathy, S. Gradient force: The mechanism for surface relief grating formation in azobenzene functionalized polymers. Appl. Phys. Lett. 1998, 72, 2096–2098. [Google Scholar] [CrossRef]
- Bian, S.; Liu, W.; Williams, J.; Samuelson, L.; Kumar, J.; Tripathy, S. Photoinduced Surface Relief Grating on Amorphous Poly(4-phenylazophenol) Films. Chem. Mater. 2000, 12, 1585–1590. [Google Scholar] [CrossRef]
- Pedersen, T.G.; Johansen, P.M.; Holme, N.C.R.; Ramanujam, P.S.; Hvilsted, S. Mean-Field Theory of Photoinduced Formation of Surface Reliefs in Side-Chain Azobenzene Polymers. Phys. Rev. Lett. 1998, 80, 89–92. [Google Scholar] [CrossRef]
- Pedersen, T.G.; Johansen, P.M. Mean-Field Theory of Photoinduced Molecular Reorientation in Azobenzene Liquid Crystalline Side-Chain Polymers. Phys. Rev. Lett. 1997, 79, 2470–2473. [Google Scholar] [CrossRef]
- Jia, J.; Sarker, M.; Steinmetz, M.G.; Shukla, R.; Rathore, R. Photochemical elimination of leaving groups from zwitterionic intermediates generated viaelectrocyclic ring closure of alpha, beta-unsaturated anilides. J. Org. Chem. 2008, 73, 8867–8879. [Google Scholar] [CrossRef]
- Jaik, T.G.; Ciubini, B.; Frascella, F.; Jonas, U. Thermal Response and Thermochromism of Methyl Red-Based Copolymer Systems—Coupled Responsiveness in Critical Solution Behaviour and Optical Absorption Properties. Polym. Chem. 2022, 13, 1186–1214. [Google Scholar] [CrossRef]
- Gianneli, M.; Roskamp, R.F.; Jonas, U.; Loppinet, B.; Fytas, G.; Knoll, W. Dynamics of swollen gel layers anchored to solid surfaces. Soft Matter 2008, 4, 1443–1447. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaik, T.G.; Flatae, A.M.; Soltani, N.; Reuschel, P.; Agio, M.; Descrovi, E.; Jonas, U. Photomotion of Hydrogels with Covalently Attached Azo Dye Moieties—Thermoresponsive and Non-Thermoresponsive Gels. Gels 2022, 8, 541. https://doi.org/10.3390/gels8090541
Jaik TG, Flatae AM, Soltani N, Reuschel P, Agio M, Descrovi E, Jonas U. Photomotion of Hydrogels with Covalently Attached Azo Dye Moieties—Thermoresponsive and Non-Thermoresponsive Gels. Gels. 2022; 8(9):541. https://doi.org/10.3390/gels8090541
Chicago/Turabian StyleJaik, Thorben G., Assegid M. Flatae, Navid Soltani, Philipp Reuschel, Mario Agio, Emiliano Descrovi, and Ulrich Jonas. 2022. "Photomotion of Hydrogels with Covalently Attached Azo Dye Moieties—Thermoresponsive and Non-Thermoresponsive Gels" Gels 8, no. 9: 541. https://doi.org/10.3390/gels8090541