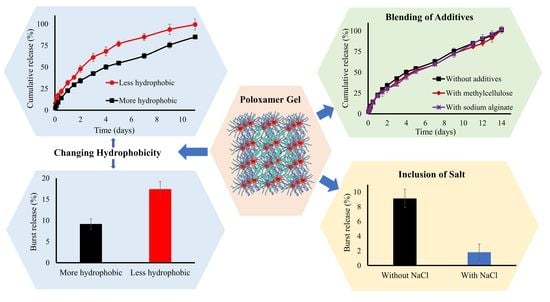
Increasing the Hydrophobic Component of Poloxamers and the Inclusion of Salt Extend the Release of Bupivacaine from Injectable In Situ Gels, While Common Polymer Additives Have Little Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Optimisation of Thermoresponsive Gel Formulations
2.3. Fourier Transform Infra-Red Spectroscopy (FTIR)
2.4. Mechanical Properties
2.4.1. Gel Strength
2.4.2. Injectability
2.5. Rheological Properties
2.5.1. Frequency Sweep
2.5.2. Flow Sweep
2.6. Development and Validation of an Analytical Method for the Determination of BH
2.7. In Vitro Drug Release Studies
2.8. Mathematical Modelling of the Release Profiles
2.9. Statistical Analysis of Data
3. Results and Discussion
3.1. Formulation Preparation and Optimisation for Sol-To-Gel Transition Temperature
3.2. Fourier Transform Infra-Red (FTIR) Spectroscopy
3.3. Mechanical Properties
3.3.1. Gel Hardness and Strength
3.3.2. Injectability
3.4. Rheological Analysis
3.4.1. Frequency Sweep
3.4.2. Flow Sweep Analysis
3.5. Analytical Method Development and Validation
3.6. In Vitro Drug Release and Kinetic Modelling Studies
3.7. Mathematical Modelling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Abdeltawab, H.; Svirskis, D.; Sharma, M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin. Drug Deliv. 2020, 17, 495–509. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Haddadi, H.; Nazockdast, E.; Ghalei, B.; Haddadi, H.; Nazockdast, E. Chemorheological characterization of thermosetting polyurethane formulations containing different chain extender contents. Polym. Eng. Sci. 2008, 48, 2446–2453. [Google Scholar] [CrossRef]
- Larsen, C.; Østergaard, J.; Larsen, S.W.; Jensen, H.; Jacobsen, S.; Lindegaard, C.; Andersen, P.H. Intra-articular depot formulation principles: Role in the management of postoperative pain and arthritic disorders. J. Pharm. Sci. 2008, 97, 4622–4654. [Google Scholar] [CrossRef]
- Sosnik, A.; Cohn, D.; Román, J.S.; Abraham, G.A. Crosslinkable PEO-PPO-PEO-based reverse thermo-responsive gels as potentially injectable materials. J. Biomater. Sci. Polym. Ed. 2003, 14, 227–239. [Google Scholar] [CrossRef]
- Jeong, B. Injectable biodegradable materials. In Injectable Biomaterials: Science and Applications; Woodhead Publishing: Cambridge, UK, 2011; pp. 323–337. [Google Scholar] [CrossRef]
- Qiu, Y.; Hamilton, S.K.; Temenoff, J. Improving mechanical properties of injectable polymers and composites. In Injectable Biomaterials: Science and Applications; Woodhead Publishing: Cambridge, UK, 2011; pp. 61–91. [Google Scholar] [CrossRef]
- Akkari, A.C.S.; Papini, J.Z.B.; Garcia, G.K.; Franco, M.K.D.; Cavalcanti, L.; Malfatti-Gasperini, A.; Alkschbirs, M.I.; Yokaichyia, F.; de Paula, E.; Tófoli, G.R.; et al. Poloxamer 407/188 binary thermosensitive hydrogels as delivery systems for infiltrative local anesthesia: Physico-chemical characterization and pharmacological evaluation. Mater. Sci. Eng. C 2016, 68, 299–307. [Google Scholar] [CrossRef]
- Ricci, E.J.J.; Lunardi, L.O.O.; Nanclares, D.M.A.M.A.; Marchetti, J.M.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Svirskis, D.; Chandramouli, K.; Bhusal, P.; Wu, Z.; Alphonso, J.; Chow, J.; Patel, D.; Ramakrishna, R.; Yeo, S.J.; Stowers, R.; et al. Injectable thermosensitive gelling delivery system for the sustained release of lidocaine. Ther. Deliv. 2016, 7, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chandramouli, K.; Curley, L.; Pontre, B.; Reilly, K.; Munro, J.; Hill, A.; Young, S.; Svirskis, D. In vitro and ex vivo characterization of an in situ gelling formulation for sustained lidocaine release with potential use following knee arthroplasty. Drug Deliv. Transl. Res. 2018, 8, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Ummadi, S.; Shravani, B.; Rao, N.R.; Reddy, M.S.; Sanjeev, B. Overview on controlled release dosage form. Int. J. Pharma. Sci. 2013, 3, 258–269. [Google Scholar]
- Lin, H.-R.R.; Sung, K.C.; Vong, W.-J.J. In Situ Gelling of Alginate/Pluronic Solutions for Ophthalmic Delivery of Pilocarpine. Biomacromolecules 2004, 5, 2358–2365. [Google Scholar] [CrossRef]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.-P.; Guo, Y.-S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.-H.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef]
- Zhang, L.; Parsons, D.L.; Navarre, C.; Kompella, U.B. Development and in-vitro evaluation of sustained release Poloxamer 407 (P407) gel formulations of ceftiofur. J. Control. Release 2002, 85, 73–81. [Google Scholar] [CrossRef]
- Yu, Z.-G.; Geng, Z.-X.; Liu, T.-F.; Jiang, F. In vitro and in vivo evaluation of an in situ forming gel system for sustained delivery of Florfenicol. J. Vet.-Pharmacol. Ther. 2015, 38, 271–277. [Google Scholar] [CrossRef]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; Abd Elhady, S.S. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007, 32, 296–307. [Google Scholar] [CrossRef]
- Sridhar, V.; Wairkar, S.; Gaud, R.; Bajaj, A.; Meshram, P. Brain targeted delivery of mucoadhesive thermosensitive nasal gel of selegiline hydrochloride for treatment of Parkinson’s disease. J. Drug Target. 2018, 26, 150–161. [Google Scholar] [CrossRef]
- Wang, W.-Y.Y.; Hui, P.C.L.; Wat, E.; Ng, F.S.F.; Kan, C.-W.; Lau, C.B.S.; Leung, P.-C. Enhanced Transdermal Permeability via Constructing the Porous Structure of Poloxamer-Based Hydrogel. Polymers 2016, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Marcos, X.; Pérez-Casas, S.; Llovo, J.; Concheiro, A.; Alvarez-Lorenzo, C. Poloxamer-hydroxyethyl cellulose-α-cyclodextrin supramolecular gels for sustained release of griseofulvin. Int. J. Pharm. 2016, 500, 11–19. [Google Scholar] [CrossRef]
- Inal, O.; Yapar, E.A. Effect of Mechanical Properties on the Release of Meloxicam from Poloxamer Gel Bases. Indian J. Pharm. Sci. 2013, 75, 700–706. [Google Scholar]
- Juhasz, J.; Lenaerts, V.; Tan, P.V.M.; Ong, H. Effect of sodium chloride on physical characteristics of poloxamer 407 solutions. J. Colloid Interface Sci. 1990, 136, 168–174. [Google Scholar] [CrossRef]
- Park, Y.-J.; Yong, C.S.; Kim, H.-M.; Rhee, J.-D.; Oh, Y.-K.; Kim, C.-K.; Choi, H.-G. Effect of sodium chloride on the release, absorption and safety of diclofenac sodium delivered by poloxamer gel. Int. J. Pharm. 2003, 263, 105–111. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Feng, L.; Yang, L.; Chen, L.; Wei, G.; Lu, W. In vivo retention of poloxamer-based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 2017, 7, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.-J.; Balakrishnan, P.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.-G. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Galgatte, U.C.; Chaudhari, P.D. Preformulation study of poloxamer 407 gels: Effect of additives. Int. J. Pharm. Sci. 2014, 6, 130–133. [Google Scholar]
- Becker, D.E.; Reed, K.L. Local Anesthetics: Review of Pharmacological Considerations. Anesth. Prog. 2012, 59, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.C.; Maniar, M. pH-Dependent solubility and dissolution of bupivacaine and its relevance to the formulation of a controlled release system. J. Control. Release 1993, 23, 261–270. [Google Scholar] [CrossRef]
- Abdeltawab, H.; Svirskis, D.; Boyd, B.J.; Hill, A.; Sharma, M. Injectable thermoresponsive gels offer sustained dual release of bupivacaine hydrochloride and ketorolac tromethamine for up to two weeks. Int. J. Pharm. 2021, 604, 120748. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm. Res. 1997, 14, 450–457. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Sun, Z.; Ali, M.A. Injectable gel from squid pen chitosan for bone tissue engineering applications. J. Sol-Gel Sci. Technol. 2016, 77, 675–687. [Google Scholar] [CrossRef]
- Zhang, Q.; Fassihi, M.A.; Fassihi, R. Delivery Considerations of Highly Viscous Polymeric Fluids Mimicking Concentrated Biopharmaceuticals: Assessment of Injectability via Measurement of Total Work Done “WT”. AAPS PharmSciTech 2018, 19, 1520–1528. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Chen, D.-W.; Quan, D.-Q. Rheological properties of poloxamer 407 aqueous solutions. Yao Xue Xue Bao = Acta Pharm. Sin. 2011, 46, 227–231. [Google Scholar]
- Guideline, I.H.T. Validation of Analytical Procedure: Text and Methodology Q2 (R1). 2005, p. 13. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 14 October 2019).
- Fathalla, Z.M.A.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterization, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Shi, X.; Lin, X.; Yao, C.; Shen, L.; Feng, Y. Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats. Drug Deliv. 2015, 22, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Cidade, M.T.; Ramos, D.J.; Santos, J.; Carrelo, H.; Calero, N.; Borges, J.P. Injectable Hydrogels Based on Pluronic/Water Systems Filled with Alginate Microparticles for Biomedical Applications. Material 2019, 12, 1083. [Google Scholar] [CrossRef] [Green Version]
- Dewan, M.; Bhowmick, B.; Sarkar, G.; Rana, D.; Bain, M.K.; Bhowmik, M.; Chattopadhyay, D. Effect of methyl cellulose on gelation behavior and drug release from poloxamer based ophthalmic formulations. Int. J. Biol. Macromol. 2015, 72, 706–710. [Google Scholar] [CrossRef]
- Majithiya, R.J.; Ghosh, P.K.; Umrethia, M.L.; Murthy, R.S.R. Thermoreversible-mucoadhesive Gel for nasal delivery of sumatriptan. AAPS PharmSciTech 2006, 7, E80–E86. [Google Scholar] [CrossRef] [Green Version]
- Khlibsuwan, R.; Khunkitti, W.; Pongjanyakul, T. Alginate-poloxamer beads for clotrimazole delivery: Molecular interactions, mechanical properties, and anticandidal activity. Int. J. Biol. Macromol. 2020, 148, 1061–1071. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Grau, R. Thermosensitive poloxamer-based injectables as controlled drug release platforms for veterinary use: Development and in-vitro evaluation. Int. Res. J. Pharm. Pharmacol. 2011, 1, 109–118. [Google Scholar]
- Zeng, N.; Dumortier, G.; Maury, M.; Mignet, N.; Boudy, V. Influence of additives on a thermosensitive hydrogel for buccal delivery of salbutamol: Relation between micellisation, gelation, mechanic and release properties. Int. J. Pharm. 2014, 467, 70–83. [Google Scholar] [CrossRef]
- Choi, H.-G.; Lee, M.-K.; Kim, M.-H.; Kim, C.-K. Effect of additives on the physicochemical properties of liquid suppository bases. Int. J. Pharm. 1999, 190, 13–19. [Google Scholar] [CrossRef]
- de Araújo, D.; dos Santos, A.C.M.; Akkari, A.C.S.; Ferreira, I.R.S.; Páscoli, M.; Guilherme, V.A.; de Paula, E.; Fraceto, L.; de Lima, R.; Melo, P.D.S.; et al. Poloxamer-based binary hydrogels for delivering tramadol hydrochloride: Sol-gel transition studies, dissolution-release kinetics, in vitro toxicity, and pharmacological evaluation. Int. J. Nanomed. 2015, 10, 2391–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero, M.; Dreiss, C.A. Growth, Shrinking, and Breaking of Pluronic Micelles in the Presence of Drugs and/or β-Cyclodextrin, a Study by Small-Angle Neutron Scattering and Fluorescence Spectroscopy. Langmuir 2010, 26, 10561–10571. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-L.; Liu, H.-Z.; Wang, J.; Chen, J.-Y. Study of Salt Effects on the Micellization of PEO–PPO–PEO Block Copolymer in Aqueous Solution by FTIR Spectroscopy. Langmuir 2002, 18, 865–871. [Google Scholar] [CrossRef]
- Jug, M.; Maestrelli, F.; Bragagni, M.; Mura, P. Preparation and solid-state characterization of bupivacaine hydrochloride cyclodextrin complexes aimed for buccal delivery. J. Pharm. Biomed. Anal. 2010, 52, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.C.; Hadgraft, J.; Bye, A.; Brookes, L.G. Drug release from Pluronic F-127 gels. Int. J. Pharm. 1986, 32, 223–228. [Google Scholar] [CrossRef]
- Rangabhatla, A.S.L.; Tantishaiyakul, V.; Oungbho, K.; Boonrat, O. Fabrication of pluronic and methylcellulose for etidronate delivery and their application for osteogenesis. Int. J. Pharm. 2016, 499, 110–118. [Google Scholar] [CrossRef]
- Cilurzo, F.; Selmin, F.; Minghetti, P.; Adami, M.; Bertoni, E.; Lauria, S.; Montanari, L. Injectability Evaluation: An Open Issue. AAPS PharmSciTech 2011, 12, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Burckbuchler, V.; Mekhloufi, G.; Giteau, A.P.; Grossiord, J.L.; Huille, S.; Agnely, F. Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. Eur. J. Pharm. Biopharm. 2010, 76, 351–356. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, H.; Liu, W.; Zou, L.; McClements, D.J. A review of the rheological properties of dilute and concentrated food emulsions. J. Texture Stud. 2020, 51, 45–55. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef]
- Baloğlu, E.; Karavana, S.Y.; Şenyiğit, Z.A.; Güneri, T. Rheological and mechanical properties of poloxamer mixtures as a mucoadhesive gel base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef]
- Yang, L.; Alexandridis, P. Controlled Release from Ordered Microstructures Formed by Poloxamer Block Copolymers. In Controlled Drug Delivery; American Chemical Society (ACS): Washington, DC, USA, 2000; pp. 364–374. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [Green Version]




| Formulation Code | Composition | Total Poloxamers Conc. (wt.%) | PPO/PEO Ratio † | Sol-To-Gel Temperature (°C), Mean ± SD |
|---|---|---|---|---|
| F1 | P407 (23%), P188 (5.5%), H2O (71.5%) | 28.5 | 0.30 | 27.0 ± 0.00 |
| F2 | P407 (20%), P188 (8.5%), H2O (71.5%) | 28.5 | 0.28 | 35.5 ± 0.33 |
| F3 | P407 (19%), P188 (5.5%), H2O (75.5%) | 24.5 | 0.25 | 40.3 ± 0.47 |
| F4 | P407 (21%), P188 (5.5%), H2O (73.5%) | 26.5 | 0.28 | 33.3 ± 0.47 |
| F5 | P407 (25%), P188 (5.5%), H2O (69.5%) | 30.5 | 0.32 | * |
| F6 | P407 (27%), P188 (5.5%), H2O (67.5%) | 33.5 | 0.34 | * |
| F1SA | Sodium alginate (0.5 wt.%) + F1 | 28.5 | 0.30 | 26.0 ± 0.00 |
| F1SA2 | Sodium alginate (0.75 wt.%) + F1 | 28.5 | 0.30 | 25.0 ± 0.33 |
| F1SA3 | Sodium alginate (1.0 wt.%) + F1 | 28.5 | 0.30 | 23.0 ± 0.33 |
| F1SA4 | Sodium alginate (0.5 wt.%) + Calcium chloride (0.5 wt.%) + F1 | 28.5 | 0.30 | ** |
| F1CMC | Carboxy methylcellulose (0.35 wt.%) + F1 | 28.5 | 0.30 | 26.3 ± 0.33 |
| F1CMC2 | Carboxy methylcellulose (0.75 wt.%) + F1 | 28.5 | 0.30 | 23.3 ± 0.33 |
| F1MC | Methylcellulose (0.35 wt.%) + F1 | 28.5 | 0.30 | 25.6 ± 0.33 |
| F1MC2 | Methylcellulose (0.5 wt.%) + F1 | 28.5 | 0.30 | 24.3 ± 0.67 |
| F1MC3 | Methylcellulose (0.75 wt.%) + F1 | 28.5 | 0.30 | 22.3 ± 0.33 |
| F1CH *** | Chitosan (MMW) (0.4 wt.%) + F1 | 28.5 | 0.30 | 25.6 ± 0.33 |
| F1CH2 *** | Chitosan (MMW) (0.5 wt.%) + F1 | 28.5 | 0.30 | 25.6 ± 0.33 |
| F1CH3 *** | Chitosan (MMW) (0.75 wt.%) + F1 | 28.5 | 0.30 | 24.3 ± 0.33 |
| F1NaCl | Sodium chloride (0.4 wt.%) + F1 | 28.5 | 0.30 | 25.4 ± 0.33 |
| F1NaCl2 | Sodium chloride (0.6 wt.%) + F1 | 28.5 | 0.30 | 24.3 ± 0.00 |
| F1NaCl3 **** | Sodium chloride (0.9 wt.%) + F1 | 28.5 | 0.30 | 22.9 ± 0.00 |
| Formulation Code | Sol-To-Gel Transition Temperature (°C) (Mean ± SD) | Sol-To-Gel Transition Time (S) (Mean ± SD) |
|---|---|---|
| F1 | 28.3 ± 0.33 | 90.0 ± 0.00 |
| F2 | 36.3 ± 0.66 | 180.0 ± 4.08 |
| F1SA | 27.3 ± 0.66 | 81.6 ± 2.35 |
| F1MC | 26.6 ± 0.00 | 83.3 ± 2.35 |
| F1CMC | 27.3 ± 0.00 | 85.0 ± 0.00 |
| F1CH | 26.6 ± 0.33 | 95.0 ± 4.08 |
| F1NaCl | 25.6 ± 0.33 | 30.0 ± 0.00 |
| Formulation Code | Gel Hardness (g) Mean ± SD | Gel Strength (g. s) Mean ± SD | Maximum Force (g) Mean ± SD | Work Needed (g. m) Mean ± SD |
|---|---|---|---|---|
| F1 | 29.0 ± 3.4 | 124.8 ± 12.8 | 1688.0 ± 55.6 | 27.5 ± 1.7 |
| F2 | 23.3 ± 1.1 | 102.3 ± 7.6 | 981.7 ± 32.0 | 15.9 ± 1.1 |
| F1SA | 40.3 ± 5.5 | 168.5 ± 23.0 | 2598.9 ± 219.8 | 47.4 ± 4.4 |
| F1MC | 39.8 ± 3.5 | 165.4 ± 13.6 | 2096.6 ± 90.5 | 37.1 ± 2.1 |
| F1CMC | 40.2 ± 2.6 | 166.8 ± 10.2 | 2526.1 ± 143.8 | 46.4 ± 2.8 |
| F1CH | 40.5 ± 4.6 | 166.2 ± 17.6 | 2448.1 ± 115.8 | 28.0 ± 1.1 |
| F1NaCl | 50.75 ± 2.7 | 207.0 ± 18.4 | 1703.6 ± 89.3 | 29.2 ± 2.1 |
| Parameters | Details |
|---|---|
| Column | Kinetex C18 ODS, 250 × 4.6 mm i.d., and 5 μm particle size |
| Column temperature | Room temperature |
| Mobile phase | Potassium phosphate buffer (pH 6.15–20 mM):acetonitrile:methanol (25:35:40) |
| Flow rate | 1.0 mL/min |
| Injection volume | 10 µL |
| Detector | Diode array detector (DAD) |
| Detection wavelength | 210 nm |
| Formulation Code | Zero-Order | First-Order | Higuchi | Hixson–Crowell | Similarity Factor (f2) |
|---|---|---|---|---|---|
| R2 | |||||
| F1 | 0.961 | 0.848 | 0.997 | 0.885 | NA |
| F2 | 0.897 | 0.980 | 0.989 | 0.991 | 43 |
| F1SA | 0.980 | 0.830 | 0.989 | 0.877 | 74 |
| F1MC | 0.971 | 0.780 | 0.992 | 0.841 | 74 |
| F1CMC | 0.977 | 0.847 | 0.992 | 0.889 | 80 |
| F1CH | 0.913 | 0.894 | 0.994 | 0.920 | 51 |
| F1NaCl | 0.949 | 0.848 | 0.993 | 0.891 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdeltawab, H.; Svirskis, D.; Hill, A.G.; Sharma, M. Increasing the Hydrophobic Component of Poloxamers and the Inclusion of Salt Extend the Release of Bupivacaine from Injectable In Situ Gels, While Common Polymer Additives Have Little Effect. Gels 2022, 8, 484. https://doi.org/10.3390/gels8080484
Abdeltawab H, Svirskis D, Hill AG, Sharma M. Increasing the Hydrophobic Component of Poloxamers and the Inclusion of Salt Extend the Release of Bupivacaine from Injectable In Situ Gels, While Common Polymer Additives Have Little Effect. Gels. 2022; 8(8):484. https://doi.org/10.3390/gels8080484
Chicago/Turabian StyleAbdeltawab, Hani, Darren Svirskis, Andrew G. Hill, and Manisha Sharma. 2022. "Increasing the Hydrophobic Component of Poloxamers and the Inclusion of Salt Extend the Release of Bupivacaine from Injectable In Situ Gels, While Common Polymer Additives Have Little Effect" Gels 8, no. 8: 484. https://doi.org/10.3390/gels8080484