Metallic Strontium as a Precursor of the Al2O3/SrCO3 Xerogels Obtained by the One-Pot Sol–Gel Method
Abstract
:1. Introduction
2. Results and Discussion
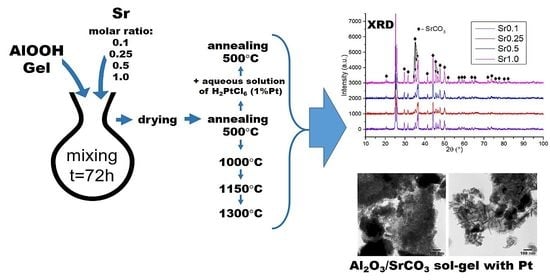
2.1. X-ray Powder Diffraction
2.2. SEM, TEM, and EDS Analysis
2.3. Porous Structure—Low Temperature Nitrogen Adsorption–Desorption
2.4. Chemisorption of Hydrogen on Pt-Al-Sr Catalysts
2.5. Thermal Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation
4.3. Characterization
4.3.1. X-ray Diffraction Analysis
4.3.2. SEM, TEM, and EDS (Energy Dispersive X-ray Spectroscopy) Analysis
4.3.3. Porous Structure
4.3.4. Thermal Analysis
4.3.5. Chemisorption of Hydrogen on Pt-Al-Sr Catalysts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuysuz, H.; Schuth, F. Chapter Two—Ordered Mesoporous Materials as Catalysts. Adv. Catal. 2012, 55, 127–239. [Google Scholar]
- Ray, S.; Gusain, R.; Kumar, N. Chapter four—Adsorption in the context of water purification. In Carbon Nanomaterial-Based Adsorbents for Water Purification; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–100. [Google Scholar]
- Kaya, G.; Deveci, H. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: A review. J. Ind. Eng. Chem. 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Iwamoto, A.; Sasaki, M.; Yoshida, K.; Aritani, H. Synthesis of SrO–Al2O3 Solid Base Catalysts from Strontium Hydroxide and Aluminum Alkoxide by a Solid-liquid Interface Reaction. J. Jpn. Pet. Inst. 2021, 64, 103–111. [Google Scholar] [CrossRef]
- Akutu, K.; Kabashima, H.; Seki, T.; Hattori, H. Nitroaldol reaction over solid base catalysts. Appl. Catal. A Gen. 2001, 247, 65–74. [Google Scholar] [CrossRef]
- Lee, H.; Wu, W.; Chen, B.H.; Liao, J.D. Heterogeneous Catalysts Using Strontium Oxide Agglomerates Depositing upon Titanium Plate for Enhancing Biodiesel Production. Catalysts 2021, 11, 30. [Google Scholar] [CrossRef]
- Busca, G. Base and Basic Materials in Chemical and Environmental Processes. Liquid Versus Solid Basicity. Chem. Rev. 2010, 110, 2217–2249. [Google Scholar] [CrossRef]
- Hibbins, S. Strontium and strontium compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; Ley, C., Ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Sezer, R.; Yilmaz, E.; Ertürk, S.; Cüneyt, A. Calcination of Strontium Carbonate in Rotary Kiln Furnace. In 10th International Symposium on High-Temperature Metallurgical Processing; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Feyzi, M.; Shahbazi, Z. Preparation, kinetic and thermodynamic studies of Al–Sr nanocatalysts for biodiesel production. J. Taiwan Inst. Chem. Eng. 2017, 71, 145–155. [Google Scholar] [CrossRef]
- Omata, K.; Nukui, N.; Hottai, T.; Showa, Y.; Yamada, M. Strontium carbonate supported cobalt catalyst for dry reforming of methane under pressure. Catal. Commun. 2004, 5, 755–758. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Omata, K.; Yamada, M. Screening of Additives to a Co/SrCO3 Catalyst by Artificial Neural Network for Preferential Oxidation of CO in Excess H2. Ind. Eng. Chem. Res. 2010, 49, 1541–1549. [Google Scholar] [CrossRef]
- Omata, K.; Kobayashi, Y.; Yamada, M. Artificial neural network-aided development of supported Co catalyst for preferential oxidation of CO in excess hydrogen. Catal. Commun. 2005, 6, 563–567. [Google Scholar] [CrossRef]
- Iida, H.; Deguchi, S.; Torigai, M.; Osawa, Y. Steam reforming of toluene over Ru/SrCO3-Al2O3 catalyst under extremely low steam-to-carbon ratio conditions. Fuel 2020, 272, 117703. [Google Scholar] [CrossRef]
- Przekop, R.; Marciniak, P.; Sztorch, B.; Czapik, A.; Stodolny, M.; Martyla, A. One-pot synthesis of Al2O3-La2O2CO3 systems obtained from the metallic precursor by the sol–gel method. J. Non-Cryst. Solids 2018, 479, 105–112. [Google Scholar] [CrossRef]
- Marciniak, P.; Sztorch, B.; Martyła, A.; Czapik, A.; Stodolny, M.; Przekop, R.E. Metallic Calcium as a Precursor for Sol–Gel Synthesis of CaCO3-SiO2 and CaO-SiO2 Systems. Ceramics 2021, 4, 278–290. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Yang, X.; Cao, X.; Hu, L.; Wang, Z.; Liu, Y.; Wang, C. Thermal investigation of strontium acetate hemihydrate in nitrogen gas. J. Therm. Anal. Calorim. 2008, 91, 169–174. [Google Scholar] [CrossRef]
- Kwon, J.O.; Seok, S.I.; Jung, D. Annealing effect on photoluminescence properties of Er doped Al2O3–SiO2 sol–gel films. J. Non-Cryst. Solids 2006, 352, 2841–2845. [Google Scholar] [CrossRef]
- Chang, Y.; Hsiang, H. Phase Evolution During Formation of SrAl2O4 from SrCO3 and Al2O3/AlOOH. J. Am. Ceram. Soc. 2007, 90, 2759–2765. [Google Scholar] [CrossRef]
- Garcés, R.S.; Torres, J.; Valdes, A.F. Synthesis of SrAl2O4 and Sr3Al2O6 at high temperature, starting from mechanically activated SrCO3 and Al2O3 in blends of 3:1 molar ratio. Ceram. Int. 2012, 38, 889–894. [Google Scholar] [CrossRef]
- Mizera; Kowalczyk, A.; Chmielarz, L.; Drożdż, E. Catalysts Based on Strontium Titanate Doped with Ni/Co/Cu for Dry Reforming of Methane. Materials 2021, 14, 7227. [Google Scholar] [CrossRef]
- Rhodes, N.; Barde, A.; Randih, K.; Li, L.; Hahn, D.; Mei, R.; Klausner, J.; AuYeyng, N. Inside Back Cover: Solar Thermochemical Energy Storage Through Carbonation Cycles of SrCO3/SrO Supported on SrZrO3. ChemSusChem 2015, 8, 3913. [Google Scholar] [CrossRef]
- Bagherisereshki, E.; Tran, J.; Lei, F.; AuYeung, N. Investigation into SrO/SrCO3 for High Temperature Thermochemical Energy Storage. Sol. Energy 2018, 160, 85–93. [Google Scholar] [CrossRef]
- Du, J.; Liu, Z.; Li, Z.; Han, B.; Huang, Y.; Zhang, J. Synthesis of mesoporous SrCO3 spheres and hollow CaCO3 spheres in room-temperature ionic liquid. Microporous Mesoporous Mater. 2005, 83, 145–149. [Google Scholar] [CrossRef]
- Przekop, R.E.; Marciniak, P.; Sztorch, B.; Czapik, A.; Stodolny, M.; Martyła, A. New method for the synthesis of Al2O3–CaO and Al2O3–CaO–CaCO3 systems from a metallic precursor by the sol–gel route. J. Aust. Ceram. Soc. 2018, 54, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Kaya, S.; Karacaoglu, E.; Karas, B. Effect of Al/Sr ratio on the luminescence properties of SrAl2O4:Eu2+, Dy3+ phosphors. Ceram. Int. 2012, 38, 3701–3706. [Google Scholar] [CrossRef]
- Liepina, V.; Smits, K.; Millers, D.; Grigorjeva, L.; Monty, C. IOP Conference Series: Materials Science and Engineering. In the Luminescent Properties of Persistent Strontium Aluminate Phosphor Prepared By Solar Induced Solid State Synthesis; IOP Publishing Ltd.: Bristol, UK, 2012. [Google Scholar]
- Przekop, R.; Marciniak, P.; Sztorch, B.; Czapik, A.; Stodolny, M.; Martyła, A. One-pot synthesis method of SiO2-La2O2CO3 andSiO2-La2O3 systems using metallic lanthanum as a precursor. J. Non-Cryst. Solids 2019, 520, 119444. [Google Scholar]
- Cunha, D.; Cruz, G. Hydrogenation of benzene and toluene over Ir particles supported on γ-Al2O3. Appl. Catal. A Gen. 2002, 236, 55–56. [Google Scholar] [CrossRef]








| System Composition | Surface Area SBET [m2/g] | Average Pore Diameter DBJH [nm] | Average Pore Volume DBJH [cm3/g] |
|---|---|---|---|
| Sr0.1 | 135 | 7 | 0.30 |
| Sr0.25 | 204 | 6 | 0.34 |
| Sr0.5 | 132 | 6 | 0.25 |
| Sr1.0 | 69 | 6 | 0.13 |
| Sr0.1 Pt | 154 | 7 | 0.29 |
| Sr0.25 Pt | 209 | 6 | 0.31 |
| Sr0.5 Pt | 159 | 7 | 0.25 |
| Sr1.0 Pt | 75 | 6 | 0.12 |
| System Composition | Metal (Pt) Dispersion [%] | Metallic Surface [m2/gmetal] | Volume of Adsorbed Hydrogen [cm3/g] |
|---|---|---|---|
| Sr0.1 Pt | 47 | 116.94 | 0.27 ± 0.006 |
| Sr0.25 Pt | 49 | 122.55 | 0.29 ± 0.004 |
| Sr0.5 Pt | 48 | 119.15 | 0.28 ± 0.001 |
| Sr1.0 Pt | 42 | 102.94 | 0.24 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanczuk-Ruszuk, E.; Sztorch, B.; Oksiuta, Z.; Przekop, R.E. Metallic Strontium as a Precursor of the Al2O3/SrCO3 Xerogels Obtained by the One-Pot Sol–Gel Method. Gels 2022, 8, 473. https://doi.org/10.3390/gels8080473
Romanczuk-Ruszuk E, Sztorch B, Oksiuta Z, Przekop RE. Metallic Strontium as a Precursor of the Al2O3/SrCO3 Xerogels Obtained by the One-Pot Sol–Gel Method. Gels. 2022; 8(8):473. https://doi.org/10.3390/gels8080473
Chicago/Turabian StyleRomanczuk-Ruszuk, Eliza, Bogna Sztorch, Zbigniew Oksiuta, and Robert E. Przekop. 2022. "Metallic Strontium as a Precursor of the Al2O3/SrCO3 Xerogels Obtained by the One-Pot Sol–Gel Method" Gels 8, no. 8: 473. https://doi.org/10.3390/gels8080473