Creating a Functional Biomimetic Cartilage Implant Using Hydrogels Based on Methacrylated Chondroitin Sulfate and Hyaluronic Acid
Abstract
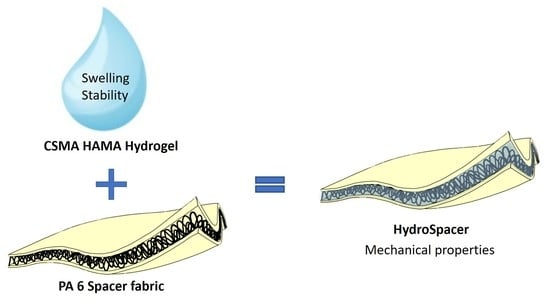
:1. Introduction
2. Results and Discussion
2.1. Biopolymer Functionalization
2.2. Hydrogel Fabrication and Crosslinking Efficiency
2.3. Hydrogel Swelling Behavior
2.4. Mechanical Characterization
2.5. Cell Viability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Functionalization of the Biopolymers
4.3. Determination of the Degree of Methacrylation with HPLC
4.4. 1H-NMR Spectroscopy
4.5. Hydrogel Fabrication
4.6. Crosslinking Efficiency Determination
4.7. Swelling
4.8. Bovine Cartilage Harvesting
4.9. Mechanical Characterization
4.10. Cell Viability
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Effect of Polyethylene Glycol on Properties and Drug Encapsulation-Release Performance of Biodegradable/Cytocompatible Agarose-Polyethylene Glycol-Polycaprolactone Amphiphilic Co-Network Gels. ACS Appl. Mater. Interfaces 2016, 8, 3182–3192. [Google Scholar] [CrossRef] [PubMed]
- Singh Chandel, A.K.; Kannan, D.; Nutan, B.; Singh, S.; Jewrajka, S.K. Dually Crosslinked Injectable Hydrogels of Poly(Ethylene Glycol) and Poly[(2-Dimethylamino)Ethyl Methacrylate]-: B -Poly(N -Isopropyl Acrylamide) as a Wound Healing Promoter. J. Mater. Chem. B 2017, 5, 4955–4965. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Mihajlovic, M.; Fermin, L.; Ito, K.; Van Nostrum, C.F.; Vermonden, T. Hyaluronic Acid-Based Supramolecular Hydrogels for Biomedical Applications. Multifunct. Mater. 2021, 4, 032001. [Google Scholar] [CrossRef]
- Igarashi, N.; Takeguchi, A.; Sakai, S.; Akiyama, H.; Higashi, K.; Toida, T. Effect of Molecular Sizes of Chondroitin Sulfate on Interaction with L-Selectin. Int. J. Carbohydr. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic Acid: A Natural Biopolymer with a Broad Range of Biomedical and Industrial Applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Chung, C.; Beecham, M.; Mauck, R.L.; Burdick, J.A. The Influence of Degradation Characteristics of Hyaluronic Acid Hydrogels on in Vitro Neocartilage Formation by Mesenchymal Stem Cells. Biomaterials 2009, 30, 4287–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Li, D.; Zhou, F.; Gao, C. Biological Hydrogel Synthesized from Hyaluronic Acid, Gelatin and Chondroitin Sulfate by Click Chemistry. Acta Biomater. 2011, 7, 1618–1626. [Google Scholar] [CrossRef]
- Ko, C.S.; Huang, J.P.; Huang, C.W.; Chu, I.M. Type II Collagen-Chondroitin Sulfate-Hyaluronan Scaffold Cross-Linked by Genipin for Cartilage Tissue Engineering. J. Biosci. Bioeng. 2009, 107, 177–182. [Google Scholar] [CrossRef]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A Biomimetic Extracellular Matrix for Cartilage Tissue Engineering Centered on Photocurable Gelatin, Hyaluronic Acid and Chondroitin Sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.F.; Oyen, M.L. On the Failure and Fracture of Hydrogels for Cartilage Replacement. J. Phys. Mater 2021, 4, 21001. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Khoneisser, J.; Huang, P.C.; Xu, X. Hydrogel as a Bioactive Material to Regulate Stem Cell Fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Benninghoff, A. Form Und Bau Der Gelenkknorpel in Ihren Beziehungen Zur Funktion—Zweiter Teil: Der Aufbau Des Gelenkknorpels in Seinen Beziehungen Zur Funktion. Zeitschrift für Zellforsch. und Mikroskopische Anat. 1925, 2, 783–862. [Google Scholar] [CrossRef]
- Grodzinsky, A.J.; Roth, V.; Myers, E.; Grossman, W.D.; Mow, V.C. The Significance of Electromechanical and Osmotic Forces in the Nonequilibrium Swelling Behavior of Articular Cartilage in Tension. J. Biomech. Eng. 1981, 103, 221–231. [Google Scholar] [CrossRef]
- Kiviranta, P.; Rieppo, J.; Korhonen, R.K.; Julkunen, P.; Töyräs, J.; Jurvelin, J.S. Collagen Network Primarily Controls Poisson’s Ratio of Bovine Articular Cartilage in Compression. J. Orthop. Res. 2006, 24, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.K.; Jurvelin, J.S. Compressive and Tensile Properties of Articular Cartilage in Axial Loading Are Modulated Differently by Osmotic Environment. Med. Eng. Phys. 2010, 32, 155–160. [Google Scholar] [CrossRef]
- Maroudas, A. Physicochemical Properties of Cartilage in the Light of Ion Exchange Theory. Biophys. J. 1968, 8, 575–595. [Google Scholar] [CrossRef] [Green Version]
- Maroudas, A. Balance between Swelling Pressure and Collagen Tension in Normal and Degenerate Cartilage. Nature 1976, 260, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Maroudas, A.; Bannon, C. Measurement of Swelling Pressure in Cartilage and Comparison with the Osmotic Pressure of Constituent Proteoglycans. Biorheology 1981, 18, 619–632. [Google Scholar] [CrossRef]
- Wilson, W.; van Donkelaar, C.C.; van Rietbergen, B.; Huiskes, R. A Fibril-Reinforced Poroviscoelastic Swelling Model for Articular Cartilage. J. Biomech. 2005, 38, 1195–1204. [Google Scholar] [CrossRef]
- Kempson, G.E.; Muir, H.; Swanson, S.A.V.; Freemas, M.A.R. Correlations between Stiffness and the Chemical Constituents of Cartilage on the Human Femoral Head. Biochim. Biophys. Acta Mol. Cell Res. 1970, 215, 70–77. [Google Scholar] [CrossRef]
- Lu, X.L.; Sun, D.D.N.; Guo, X.E.; Chen, F.H.; Lai, W.M.; Mow, V.C. Indentation Determined Mechanoelectrochemical Properties and Fixed Charge Density of Articular Cartilage. Ann. Biomed. Eng. 2004, 32, 370–379. [Google Scholar] [CrossRef]
- Lux Lu, X.; Miller, C.; Chen, F.H.; Edward Guo, X.; Mow, V.C. The Generalized Triphasic Correspondence Principle for Simultaneous Determination of the Mechanical Properties and Proteoglycan Content of Articular Cartilage by Indentation. J. Biomech. 2007, 40, 2434–2441. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sakai, N.; Sawae, Y.; Tanaka, K.; Ihari, M. Influence of Proteoglycan on Time-Dependent Mechanical Behaviors of Articular Cartilage under Constant Total Compressive Deformation. JSME Int. J. Ser. C 2004, 47, 1049–1055. [Google Scholar] [CrossRef]
- Canal Guterl, C.; Hung, C.T.; Ateshian, G.A. Electrostatic and Non-Electrostatic Contributions of Proteoglycans to the Compressive Equilibrium Modulus of Bovine Articular Cartilage. J. Biomech. 2010, 43, 1343–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korhonen, R.K.; Laasanen, M.S.; Töyräs, J.; Lappalainen, R.; Helminen, H.J.; Jurvelin, J.S. Fibril Reinforced Poroelastic Model Predicts Specifically Mechanical Behavior of Normal, Proteoglycan Depleted and Collagen Degraded Articular Cartilage. J. Biomech. 2003, 36, 1373–1379. [Google Scholar] [CrossRef]
- Lu, X.L.; Mow, V.C. Biomechanics of Articular Cartilage and Determination of Material Properties. Med. Sci. Sports Exerc. 2008, 40, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mow, V.C.; Ateshian, G.A.; Lai, W.M.; Gu, W.Y. Effects of Fixed Charges on the Stress-Relaxation Behavior of Hydrated Soft Tissues in a Confined Compression Problem. Int. J. Solids Struct. 1998, 35, 4945–4962. [Google Scholar] [CrossRef]
- Räsänen, L.P.; Tanska, P.; Zbýň, Š.; Van Donkelaar, C.C.; Trattnig, S.; Nieminen, M.T.; Korhonen, R.K. The Effect of Fixed Charge Density and Cartilage Swelling on Mechanics of Knee Joint Cartilage during Simulated Gait. J. Biomech. 2017, 61, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.; Van Donkelaar, C.C.; Van Rietbergen, B.; Ito, K.; Huiskes, R. Stresses in the Local Collagen Network of Articular Cartilage: A Poroviscoelastic Fibril-Reinforced Finite Element Study. J. Biomech. 2004, 37, 357–366. [Google Scholar] [CrossRef]
- Quiroga, J.M.P.; Wilson, W.; Ito, K.; van Donkelaar, C.C. Relative Contribution of Articular Cartilage’s Constitutive Components to Load Support Depending on Strain Rate. Biomech. Model. Mechanobiol. 2017, 16, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Schuurmans, C.C.L.; Mihajlovic, M.; Hiemstra, C.; Ito, K.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid and Chondroitin Sulfate (Meth)Acrylate-Based Hydrogels for Tissue Engineering: Synthesis, Characteristics and Pre-Clinical Evaluation. Biomaterials 2021, 268, 120602. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, T.; Xiao, Z. Hydrogels of Collagen / Chondroitin Sulfate / Hyaluronan Interpenetrating Polymer Network for Cartilage Tissue Engineering. J. Mater. Sci. Mater. Med. 2012, 2267–2279. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, Q.; Sun, Y.; Li, G.; Bian, L. Effect of Cartilaginous Matrix Components on the Chondrogenesis and Hypertrophy of Mesenchymal Stem Cells in Hyaluronic Acid Hydrogels. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.A.; Hutmacher, D.W.; Malda, J.; Klein, T.J. Hyaluronic Acid Enhances the Mechanical Properties of Tissue-Engineered Cartilage Constructs. PLoS ONE 2014, 9, e113216. [Google Scholar] [CrossRef]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D Bioprinting of BM-MSCs-Loaded ECM Biomimetic Hydrogels for in Vitro Neocartilage Formation. Biofabrication 2016, 8, 035002. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.C.; Moroni, L.; Barrias, C.C.; Granja, P.L. Leveling Up Hydrogels: Hybrid Systems in Tissue Engineering. Trends Biotechnol. 2020, 38, 292–315. [Google Scholar] [CrossRef]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of Hydrogels Using Three-Dimensionally Printed Microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef]
- Schäfer, B.; Emonts, C.; Glimpel, N.; Ruhl, T.; Obrecht, A.S.; Jockenhoevel, S.; Gries, T.; Beier, J.P.; Blaeser, A. Warp-Knitted Spacer Fabrics: A Versatile Platform to Generate Fiber-Reinforced Hydrogels for 3D Tissue Engineering. Materials 2020, 13, 3518. [Google Scholar] [CrossRef]
- Schuiringa, G.H.; Ito, K.; Van Donkelaar, C.C. The Osmotic Swelling in HydroSpacers Can Be Used to Create Load-Bearing Cartilage Implants. In Proceedings of the 26th Congress of the European Society of Biomechanics, Milan, Italy, 11–14 July 2021. [Google Scholar]
- Abbadessa, A.; Blokzijl, M.M.; Mouser, V.H.M.; Marica, P.; Malda, J.; Hennink, W.E.; Vermonden, T. A Thermo-Responsive and Photo-Polymerizable Chondroitin Sulfate-Based Hydrogel for 3D Printing Applications. Carbohydr. Polym. 2016, 149, 163–174. [Google Scholar] [CrossRef]
- Abbadessa, A.; Mouser, V.H.M.; Blokzijl, M.M.; Gawlitta, D.; Dhert, W.J.A.; Hennink, W.E.; Malda, J.; Vermonden, T. A Synthetic Thermosensitive Hydrogel for Cartilage Bioprinting and Its Biofunctionalization with Polysaccharides. Biomacromolecules 2016, 17, 2137–2147. [Google Scholar] [CrossRef] [Green Version]
- Schuurmans, C.C.L.; Brouwer, A.J.; Jong, J.A.W.; Boons, G.J.P.H.; Hennink, W.E.; Vermonden, T. Hydrolytic (In)Stability of Methacrylate Esters in Covalently Cross-Linked Hydrogels Based on Chondroitin Sulfate and Hyaluronic Acid Methacrylate. ACS Omega 2021, 6, 26302–26310. [Google Scholar] [CrossRef]
- De Groot, C.J.; Van Luyn, M.J.A.; Van Dijk-Wolthuis, W.N.E.; Cadée, J.A.; Plantinga, J.A.; Den Otter, W.; Hennink, W.E. In Vitro Biocompatibility of Biodegradable Dextran-Based Hydrogels Tested with Human Fibroblasts. Biomaterials 2001, 22, 1197–1203. [Google Scholar] [CrossRef]
- Esterbauer, H.; Zollner, H.; Scholz, N. Reaction of Glutathione with Conjugated Carbonyls. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 1975, 30, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.; Weinstein, H.; Namboodiri, K.; Rabinowitz, J.R. Reactivities of Acrylic and Methacrylic Acids in a Nucleophilic Addition Model of Their Biological Activity. J. Am. Chem. Soc. 1988, 110, 1701–1707. [Google Scholar] [CrossRef]
- Kurata, S.; Morishita, K.; Kawase, T.; Umemoto, K. Cytotoxic Effects of Acrylic Acid, Methacrylic Acid, Their Corresponding Saturated Carboxylic Acids, HEMA, and Hydroquinone on Fibroblasts Derived from Human Pulp. Dent. Mater. J. 2012, 31, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Wetering, P.; Zuidam, N.J.; Van Steenbergen, M.J.; Van Der Houwen, O.A.G.J.; Underberg, W.J.M.; Hennink, W.E. A Mechanistic Study of the Hydrolytic Stability of Poly(2-(Dimethylamino)Ethyl Methacrylate). Macromolecules 1998, 31, 8063–8068. [Google Scholar] [CrossRef]
- Van Dijk-Wolthuis, W.N.E.; Van Steenbergen, M.J.; Underberg, W.J.M.; Hennink, W.E. Degradation Kinetics of Methacrylated Dextrans in Aqueous Solution. J. Pharm. Sci. 1997, 86, 413–417. [Google Scholar] [CrossRef]
- Van Dijk-Wolthuis, W.N.E.; Hoogeboom, J.A.M.; Van Steenbergen, M.J.; Tsang, S.K.Y.; Hennink, W.E. Degradation and Release Behavior of Dextran-Based Hydrogels. Macromolecules 1997, 30, 4639–4645. [Google Scholar] [CrossRef]
- Pichert, A.; Samsonov, S.A.; Theisgen, S.; Thomas, L.; Baumann, L.; Schiller, J.; Beck-Sickinger, A.G.; Huster, D.; Pisabarro, M.T. Characterization of the Interaction of Interleukin-8 with Hyaluronan, Chondroitin Sulfate, Dermatan Sulfate and Their Sulfated Derivatives by Spectroscopy and Molecular Modeling. Glycobiology 2012, 22, 134–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, S.; Hwang, N.S.; Canver, A.C.; Theprungsirikul, P.; Lin, D.W.; Elisseeff, J. Chondroitin Sulfate Based Niches for Chondrogenic Differentiation of Mesenchymal Stem Cells. Matrix Biol. 2008, 27, 12–21. [Google Scholar] [CrossRef]
- Lee, C.T.; Kung, P.H.; Lee, Y. Der Preparation of Poly(Vinyl Alcohol)-Chondroitin Sulfate Hydrogel as Matrices in Tissue Engineering. Carbohydr. Polym. 2005, 61, 348–354. [Google Scholar] [CrossRef]
- Martens, P.; Metters, A.T.; Anseth, K.S.; Bowman, C.N. A Generalized Bulk-Degradation Model for Hydrogel Networks Formed from Multivinyl Cross-Linking Molecules. J. Phys. Chem. B 2001, 105, 5131–5138. [Google Scholar] [CrossRef]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled Degradation and Mechanical Behavior of Photopolymerized Hyaluronic Acid Networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poldervaart, M.T.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Öner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D Bioprinting of Methacrylated Hyaluronic Acid (MeHA) Hydrogel with Intrinsic Osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach, J.B.; Bivens, K.A.; Patrick, C.W.; Schmidt, C.E. Photocrosslinked Hyaluronic Acid Hydrogels: Natural, Biodegradable Tissue Engineering Scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef]
- Hachet, E.; Van Den Berghe, H.; Bayma, E.; Block, M.R.; Auzély-Velty, R. Design of Biomimetic Cell-Interactive Substrates Using Hyaluronic Acid Hydrogels with Tunable Mechanical Properties. Biomacromolecules 2012, 13, 1818–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjmandi, M.; Ramezani, M.; Bolle, T.; Köppe, G.; Gries, T.; Neitzert, T. Mechanical and Tribological Properties of a Novel Hydrogel Composite Reinforced by Three-Dimensional Woven Textiles as a Functional Synthetic Cartilage. Compos. Part A Appl. Sci. Manuf. 2018, 115, 123–133. [Google Scholar] [CrossRef]
- Shinzawa, H.; Mizukado, J. Water Absorption by Polyamide (PA) 6 Studied with Two-Trace Two-Dimensional (2T2D) near-Infrared (NIR) Correlation Spectroscopy. J. Mol. Struct. 2020, 1217, 128389. [Google Scholar] [CrossRef]
- Valonen, P.K.; Moutos, F.T.; Kusanagi, A.; Moretti, M.G.; Diekman, B.O.; Welter, J.F.; Caplan, A.I.; Guilak, F.; Freed, L.E. In Vitro Generation of Mechanically Functional Cartilage Grafts Based on Adult Human Stem Cells and 3D-Woven Poly(ε-Caprolactone) Scaffolds. Biomaterials 2010, 31, 2193–2200. [Google Scholar] [CrossRef] [Green Version]
- Moutos, F.T.; Glass, K.A.; Compton, S.A.; Ross, A.K.; Gersbach, C.A.; Guilak, F.; Estes, B.T. Anatomically Shaped Tissue-Engineered Cartilage with Tunable and Inducible Anticytokine Delivery for Biological Joint Resurfacing. Proc. Natl. Acad. Sci. USA 2016, 113, E4513–E4522. [Google Scholar] [CrossRef] [Green Version]
- Athanasiou, K.A.; Rosenwasser, M.P.; Buckwalter, J.A.; Malinin, T.I.; Mow, V.C. Interspecies Comparisons of in Situ Intrinsic Mechanical Properties of Distal Femoral Cartilage. J. Orthop. Res. 1991, 9, 330–340. [Google Scholar] [CrossRef]
- Jurvelin, J.S.; Buschmann, M.D.; Hunziker, E.B. Mechanical Anisotropy of the Human Knee Articular Cartilage in Compression. Proc. Inst. Mech. Eng. H. 2003, 217, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.K.; Laasanen, M.S.; Toyras, J.; Rieppo, J.; Hirvonen, J.; Helminen, H.J.; Juvelin, J. Comparison of the Equilibrium Reponse Fo AC in Unconfined Compression and Indentation. J. Biomech. 2002, 35, 903–909. [Google Scholar] [CrossRef]
- Froimson, M.I.; Ratcliffe, A.; Gardner, T.R.; Mow, V.C. Differences in Patellofemoral Joint Cartilage Material Properties and Their Significance to the Etiology of Cartilage Surface Fibrillation. Osteoarthr. Cartil. 1997, 5, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, B.; Spitz, S.; Schädl, B.; Teuschl, A.H.; Redl, H.; Nürnberger, S.; Ertl, P. Stiffness Matters: Fine-Tuned Hydrogel Elasticity Alters Chondrogenic Redifferentiation. Front. Bioeng. Biotechnol. 2020, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Mouser, V.; Chen, M.; Malda, J.; Ito, K. Bi-Layered Micro-Fibre Reinforced Hydrogels for Articular Cartilage Regeneration. Acta Biomater. 2019, 95, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Hayami, J.W.S.; Waldman, S.D.; Amsden, B.G. Photo-Cross-Linked Methacrylated Polysaccharide Solution Blends with High Chondrocyte Viability, Minimal Swelling, and Moduli Similar to Load Bearing Soft Tissues. Eur. Polym. J. 2015, 72, 687–697. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Rikkers, M.; Mihajlovic, M.; Viola, M.; Schuiringa, G.; Ilochonwu, B.C.; Masereeuw, R.; Vonk, L.; Malda, J.; Ito, K.; et al. Viscoelastic Chondroitin Sulfate and Hyaluronic Acid Double-Network Hydrogels with Reversible Cross-Links. Biomacromolecules 2022, 23, 1350–1365. [Google Scholar] [CrossRef]
- Stenekes, R.J.H.; Hennink, W.E. Polymerization Kinetics of Dextran-Bound Methacrylate in an Aqueous Two Phase System. Polymer 2000, 41, 5563–5569. [Google Scholar] [CrossRef]






| Sample a | Spacer Fabric | LAP (w/v%) | Exposure Duration (min) | UV Exposure (Direction) | UV Intensity (mW/cm2) |
|---|---|---|---|---|---|
| CSMA/HAMA | No | 0.3 | 15 | One side | 0.58 |
| CSMA/HAMA | No | 0.2 | 15 (7.5 each side) | Both sides | 1.49 |
| CSMA/HAMA | No | 0.3 | 15 (7.5 each side) | Both sides | 1.49 |
| CSMA/HAMA/PA6 | Yes | 0.3 | 15 | One side | 0.58 |
| CSMA/HAMA/PA6 | Yes | 0.2 | 15 | Both sides | 1.49 |
| CMSA/HAMA/PA6 | Yes | 0.3 | 15 (7.5 each side) | Both sides | 1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuiringa, G.H.; Mihajlovic, M.; van Donkelaar, C.C.; Vermonden, T.; Ito, K. Creating a Functional Biomimetic Cartilage Implant Using Hydrogels Based on Methacrylated Chondroitin Sulfate and Hyaluronic Acid. Gels 2022, 8, 457. https://doi.org/10.3390/gels8070457
Schuiringa GH, Mihajlovic M, van Donkelaar CC, Vermonden T, Ito K. Creating a Functional Biomimetic Cartilage Implant Using Hydrogels Based on Methacrylated Chondroitin Sulfate and Hyaluronic Acid. Gels. 2022; 8(7):457. https://doi.org/10.3390/gels8070457
Chicago/Turabian StyleSchuiringa, Gerke H., Marko Mihajlovic, Corrinus C. van Donkelaar, Tina Vermonden, and Keita Ito. 2022. "Creating a Functional Biomimetic Cartilage Implant Using Hydrogels Based on Methacrylated Chondroitin Sulfate and Hyaluronic Acid" Gels 8, no. 7: 457. https://doi.org/10.3390/gels8070457