A Novel Method for the Preparation of Poly (Acrylamide-co-Acrylonitrile) Upper Critical Solution Temperature Thermosensitive Hydrogel by the Partial Dehydration of Acrylamide Grafted Polypropylene Sheets
Abstract
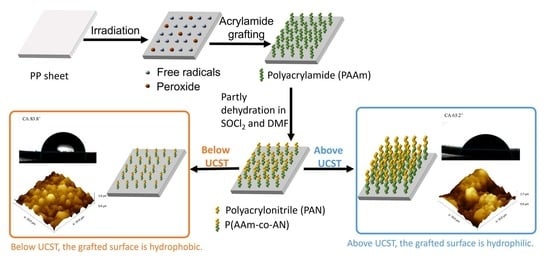
:1. Introduction
2. Experiment
2.1. Chemicals and Materials
2.2. Irradiation
2.3. Grafting Procedure
2.4. Dehydration
2.5. Analytical Techniques
2.5.1. ATR-FTIR Spectroscopy
2.5.2. Contact Angle Test
2.5.3. Atomic Force Microscope (AFM)
2.5.4. Quartz Crystal Microbalance (QCM)
2.5.5. Cytotoxicity Test
3. Results and Discussion
3.1. Grafting Reaction
3.2. Effects of the Absorbed Dose of the Pre-Irradiated PP Sheet, Reaction Temperature, and Monomer Concentration on the Degree of Grafting
3.3. Dehydration of PP-g-PAAm into PP-g-P (AAm-co-AN)
3.4. Effect of the SOCl2 Ratio in DMF/SOCl2 on the Dehydration of AAm
4. UCST Test
5. Cytotoxicity Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; da Silva, L.P.; Pirraco, R.P.; Ma, M.; Yang, L.; Reis, R.L.; Chen, J. A thermo-/pH-responsive hydrogel (PNIPAM-PDMA-PAA) with diverse nanostructures and gel behaviors as a general drug carrier for drug release. Polym. Chem. 2018, 9, 4063–4072. [Google Scholar] [CrossRef]
- Reed, J.A.; Lucero, A.E.; Hu, S.; Ista, L.K.; Bore, M.T.; López, G.P.; Canavan, H.E. A Low-Cost, Rapid Deposition Method for "Smart" sheets: Applications in Mammalian Cell Release. ACS Appl. Mater. Interfaces 2010, 2, 1048–1051. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of Cell Detachment from Temperature-Modulated, Hydrophilic-Hydrophobic Polymer Surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Canavan, H.E.; Cheng, X.; Graham, D.J.; Ratner, B.D.; Castner, D.G. Surface Characterization of the Extracellular Matrix Remaining after Cell Detachment from a Thermoresponsive Polymer. Langmuir 2004, 21, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, K.; Wischerhoff, E.; Lutz, J.F.; Laschewsky, A.; Jaeger, M.S.; Lankenau, A.; Duschl, C. Monitoring cell detachment on PEG-based thermoresponsive surfaces using TIRF microscopy. Soft Matter 2010, 6, 4262–4267. [Google Scholar] [CrossRef]
- Cunliffe, D.; de las Heras Alarcón, C.; Peters, V.; Smith, J.R.; Alexander, C. Thermoresponsive Surface-Grafted Poly (N-isopropylacrylamide) Copolymers: Effect of Phase Transitions on Protein and Bacterial Attachment. Langmuir 2003, 19, 2888–2899. [Google Scholar] [CrossRef]
- A1-Jamal, W.T.; AI—Ahmady, Z.S.; Kostarelos, K. Pharmacokinetics & Tissue Distribution of Temperature—Sensitive Liposomal Doxorubicin in Tumor-Bearing Mice Triggered with Mid Hyperthermia. Biomaterials 2012, 33, 4608–4617. [Google Scholar]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, L.Y.; Cheng, C.J.; Mi, D.F.; Zhou, M.Y.; Ju, X.J. Graft-type poly (N-isopropylacrylamide-co-acrylic acid) microgels exhibiting rapid thermo- and pH-responsive properties. Polymer 2008, 49, 2595–2603. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Licea-Claverie, A.; Cornejo-Bravo, J.M.; Schwarz, S.; Arndt, K.F. Well-defined N-Isopropylacrylamide Dual-Sensitive Copolymers with LCST ≈ 38 ℃ in Different Architectures: Linear, Block and Star Polymers. Macromol. Chem. Phys. 2012, 213, 301–314. [Google Scholar] [CrossRef]
- Tan, B.; Pelton, R.; Tam, K. Microstructure and rheological properties of thermo-responsive poly(N-isopropylacrylamide) microgels. Polymer 2010, 51, 3238–3243. [Google Scholar] [CrossRef]
- Xue, X.; Thiagarajan, L.; Braim, S.; Saunders, B.R.; Shakesheff, K.M.; Alexander, C. Upper critical solution temperature thermo-responsive polymer brushes and a mechanism for controlled cell attachment. J. Mater. Chem. B 2017, 5, 4926–4933. [Google Scholar] [CrossRef] [PubMed]
- Aseyev, V.O.; Tenhu, H.; Winnik, F.M. Temperature dependence of the colloidal stability of neutral amphiphilic polymers in water. Adv. Polym. Sci. 2011, 242, 29–89. [Google Scholar]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution: Unexpected Properties from Known Building Blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Haas, H.C.; Schuler, N.W. Thermally reversible homopolymer gel systems. J. Polym. Sci. Part B Polym. Lett. 1964, 2, 1095–1096. [Google Scholar] [CrossRef]
- Brahme, N.M.; Smith, W.T. Synthesis of some graft polymers of uracil. Polym. Chem. Ed. 1984, 22, 813–820. [Google Scholar] [CrossRef]
- Liu, F.; Seuring, J.; Agarwal, S. Atom transfer radical polymerization as a tool for making poly(N-acryloylglycinamide) with molar mass independent UCST-type transitions in water and electrolytes. Polym. Chem. 2013, 4, 3123–3131. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. First Example of a Universial and Cost-Effective Approach: Polymers with Tunable Upper Critical Solution Temperature in Watter and Electrolyte Solution. Macromolecules 2012, 45, 3910–3918. [Google Scholar] [CrossRef]
- Asadujjaman, A.; Kent, B.; Bertin, A. Phase transition and aggregation behaviour of an UCST-type copolymer poly(acrylamide-co-acrylonitrile) in water: Effect of acrylonitrile content, concentration in solution, copolymer chain length and presence of electrolyte. Soft Matter 2017, 13, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tong, X.; Zhao, Y. Diverse thermoresponsive behaviors of uncharged UCST block copolymer micelles in physiological medium. Langmuir 2014, 30, 11433–11441. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Zhao, B. Thermoreversible Physically Crosslinked Hydrogels from UCST-Type Thermosensitive ABA Linear Triblock Copolymers. Polym. Chem. 2016, 7, 6980–6991. [Google Scholar] [CrossRef]
- Chen, L.; Yang, T.; Niu, Y.; Mu, X.; Gong, Y.; Feng, Y.; de Rooij, N.F.; Wang, Y.; Li, H.; Zhou, G. Building a smart surface with converse temperature-dependent wettability based on poly(acrylamide-co-acrylonitrile). Chem. Commun. 2020, 56, 2837–2840. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.M.; Wu, M.H.; Xi, Q.; He, S.; Li, Y.; Nho, Y.C. Preparation of interpenetrating polymer networks by two times grafting of monomers onto preirradiated polypropylene sheet. Radiat. Phys. Chem. 2000, 59, 313–316. [Google Scholar] [CrossRef]
- Chen, J.; Nho, Y.C.; Park, J.S. Grafting polymerization of acrylic Acid onto preirradiated polypropylene fabric. Radiat. Phys. Chem. 1998, 52, 201–206. [Google Scholar] [CrossRef]
- Hui, B.; Chen, J.; Yang, L.; Li, J.; Pei, Y.; Shi, L. Preparation of pH sensitive hydrogel by two times grafting of acrylamide and acrylic acid onto preirradiated polyethylene film. J. Radio Anal. Nucl. Chem. 2004, 260, 673–677. [Google Scholar] [CrossRef]
- Su, W.; Weng, Y.; Jiang, L.; Yang, Y.; Zhao, L.; Chen, Z.; Li, Z.; Li, J. Recent Progress in the Use of Vilsmeier-Type Reagents. Cheminform 2011, 42, 503–555. [Google Scholar] [CrossRef]












| AN (%) | UCST (°C) |
|---|---|
| 16.22 | 25.5 |
| 20.14 | 30.5 |
| 22.86 | 32.5 |
| 26.53 | 40.5 |
| 30.66 | 49.5 |
| 31.93 | 54.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, Y.; Chen, L.; Huang, M.; Zhou, C.; Yang, L.; Niu, H.; Su, L.; Yang, Y.; Pirraco, R.P.; Reis, R.L.; et al. A Novel Method for the Preparation of Poly (Acrylamide-co-Acrylonitrile) Upper Critical Solution Temperature Thermosensitive Hydrogel by the Partial Dehydration of Acrylamide Grafted Polypropylene Sheets. Gels 2022, 8, 345. https://doi.org/10.3390/gels8060345
Ling Y, Chen L, Huang M, Zhou C, Yang L, Niu H, Su L, Yang Y, Pirraco RP, Reis RL, et al. A Novel Method for the Preparation of Poly (Acrylamide-co-Acrylonitrile) Upper Critical Solution Temperature Thermosensitive Hydrogel by the Partial Dehydration of Acrylamide Grafted Polypropylene Sheets. Gels. 2022; 8(6):345. https://doi.org/10.3390/gels8060345
Chicago/Turabian StyleLing, Yi, Liuyuchen Chen, Mingjun Huang, Cheng Zhou, Liming Yang, Hejingying Niu, Li Su, Yuejiao Yang, Rogério P. Pirraco, Rui L. Reis, and et al. 2022. "A Novel Method for the Preparation of Poly (Acrylamide-co-Acrylonitrile) Upper Critical Solution Temperature Thermosensitive Hydrogel by the Partial Dehydration of Acrylamide Grafted Polypropylene Sheets" Gels 8, no. 6: 345. https://doi.org/10.3390/gels8060345