A Temperature-Sensitive Polymeric Rheology Modifier Used in Water-Based Drilling Fluid for Deepwater Drilling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of PNBAM
2.1.1. FT-IR
2.1.2. H-NMR Spectra
2.1.3. TGA
2.2. Performance of PNBAM
2.2.1. Rheological Performance of PNBAM
2.2.2. Temperature Resistance Analysis
2.2.3. Salt and Calcium Resistance Analysis
2.2.4. Performance of Drilling Fluid System
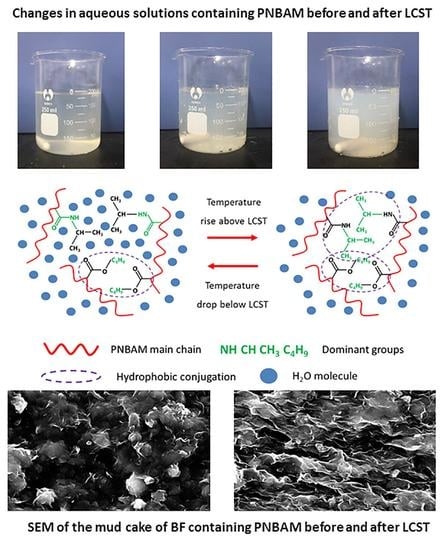
2.3. Mechanism Study
2.3.1. Influencing Factors of LCST
2.3.2. Particle Size Analysis
2.3.3. Zeta Potential Analysis
2.3.4. Mechanism Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Experimental Methods
4.2.1. Synthesis of Rheology Modifier PNBAM
4.2.2. Characterization
- FT-IR
- 2.
- TGA
- 3.
- 1H-NMR spectra
4.2.3. Performance Evaluation
- Low temperature rheological performance tests
- 2.
- Temperature resistance tests
- 3.
- Salt and calcium resistance tests
- 4.
- Drilling fluid system tests
4.2.4. Mechanism Analysis
- LCST
- 2.
- Effect of PNBAM concentration on LCST
- 3.
- Effect of salt concentration on LCST
- 4.
- Particle size
- 5.
- Zeta potential
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kjärstad, J.; Johnsson, F. Resources and future supply of oil. Energy Policy 2009, 37, 441–464. [Google Scholar] [CrossRef]
- Clerici, A.; Alimonti, G. World energy resources. In Proceedings of the EPJ WEB of Conferences, Milano, Italy, 27 August 2015; p. 01001. [Google Scholar]
- Pinder, D. Offshore oil and gas: Global resource knowledge and technological change. Ocean. Coast. Manag. 2001, 44, 579–600. [Google Scholar] [CrossRef]
- Altun, N.E.; Hicyilmaz, C.; Hwang, J.-Y.; Suat BaǦci, A.; Kök, M.V. Oil shales in the world and turkey, reserves, current situation and future prospects: A review. Oil Shale 2006, 23, 211–227. [Google Scholar]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Cui, D.; Yin, H.; Liu, Y.; Li, J.; Pan, S.; Wang, Q. Effect of final pyrolysis temperature on the composition and structure of shale oil: Synergistic use of multiple analysis and testing methods. Energy 2022, 252, 124062. [Google Scholar] [CrossRef]
- Hu, S.; Wu, H.; Liang, X.; Xiao, C.; Zhao, Q.; Cao, Y.; Han, X. A preliminary study on the eco-environmental geological issue of in-situ oil shale mining by a physical model. Chemosphere 2022, 287, 131987. [Google Scholar] [CrossRef]
- Huang, K.; Su, B.; Li, T.; Ke, H.; Lin, M.; Wang, Q. Numerical simulation of the mixing behaviour of hot and cold fluids in the rectangular T-junction with/without an impeller. Appl. Therm. Eng. 2022, 204, 117942. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Dai, L.; Liu, Q.; Fang, D.; Wei, A.; Wang, J. Friction-wear failure mechanism of tubing strings used in high-pressure, high-temperature and high-yield gas wells. Wear 2021, 468, 203576. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, G.; Zhou, J.; Li, H.; Shi, L.; Xu, X.; Cheng, B.; Zhuang, X. Proton Donor-Regulated Mechanically Robust Aramid Nanofiber Aerogel Membranes for High-Temperature Thermal Insulation. ACS Nano 2022, 16, 5984–5993. [Google Scholar] [CrossRef]
- Davies, A.J.; Roberts, J.M.; Hall-Spencer, J. Preserving deep-sea natural heritage: Emerging issues in offshore conservation and management. Biol. Conserv. 2007, 138, 299–312. [Google Scholar] [CrossRef]
- Klueh, U.H.; Pastor, G.; Segura, A. Policies to improve the local impact from hydrocarbon extraction: Observations on West Africa and possible lessons for Central Asia. Energy Policy 2009, 37, 1128–1144. [Google Scholar] [CrossRef]
- Hong, Z. The South China sea dispute and China-ASEAN relations. Asian Aff. 2013, 44, 27–43. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Gu, Y.; Yang, S.; Tang, Z.; Sun, Y.; Wu, Q. Techno-economic evaluation of CO2-rich natural gas dry reforming for linear alpha olefins production. Energy Convers. Manag. 2020, 205, 112348. [Google Scholar] [CrossRef]
- Burgess, J.P. The politics of the South China Sea: Territoriality and international law. Secur. Dialogue 2003, 34, 7–10. [Google Scholar] [CrossRef]
- Shicun, W.; Nong, H. The energy security of China and oil and gas exploitation in the South China Sea. In Recent Developments in the Law of the Sea and China; Brill Nijhoff: Leiden, The Netherlands, 2006; pp. 145–154. [Google Scholar]
- Hu, W.; Bao, J.; Hu, B. Trend and progress in global oil and gas exploration. Pet. Explor. Dev. 2013, 40, 439–443. [Google Scholar] [CrossRef]
- Zamora, M.; Broussard, P.; Stephens, M. The top 10 mud-related concerns in deepwater drilling operations. In Proceedings of the SPE International Petroleum Conference and Exhibition in Mexico, Villahermosa, Mexico, 1 February 2000. [Google Scholar]
- Shah, S.N.; Shanker, N.H.; Ogugbue, C.C. Future challenges of drilling fluids and their rheological measurements. In Proceedings of the AADE Fluids Conference and Exhibition, Houston, TX, USA, 6–7 April 2010. [Google Scholar]
- Van Oort, E.; Lee, J.; Friedheim, J.; Toups, B. New flat-rheology synthetic-based mud for improved deepwater drilling. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26 September 2004. [Google Scholar]
- Zhao, X.; Qiu, Z.; Huang, W.; Wang, M. Mechanism and method for controlling low-temperature rheology of water-based drilling fluids in deepwater drilling. J. Pet. Sci. Eng. 2017, 154, 405–416. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, X.; Li, Y.; Liu, W.; Luo, M. Application a novel thermo-sensitive copolymer as a potential rheological modifier for deepwater water-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123848. [Google Scholar] [CrossRef]
- Xu, L.; Xu, M.-B.; Zhao, L.; Wen, S.-C.; Liu, W.-H.; Xu, J.; You, F.-C.; Gong, C. Experimental investigations into the performance of a flat-rheology water-based drilling fluid. SPE J. 2014, 19, 69–77. [Google Scholar] [CrossRef]
- Said, M.M.; El-Sayed, A.A.H. The use of palm oil fatty acid methyl ester as a base fluid for a flat rheology high-performance drilling fluid. J. Pet. Sci. Eng. 2018, 166, 969–983. [Google Scholar] [CrossRef]
- Young, S.; Friedheim, J.; Lee, J.; Prebensen, O.I. A new generation of flat rheology invert drilling fluids. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 28 March 2012. [Google Scholar]
- Hilfiger, M.G.; Thaemlitz, C.J.; Moellendick, E. Investigating the chemical nature of flat rheology. In Proceedings of the SPE Deepwater Drilling and Completions Conference, Galveston, TX, USA, 14 September 2016. [Google Scholar]
- Jiang, G.; He, S.; Yinbo, H.E. The biodiesel-based flat-rheology drilling fluid system. Pet. Explor. Dev. 2022, 49, 200–210. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Xu, J.; Zhao, C.; Gao, J. Flat-rheology oil-based drilling fluid for deepwater drilling. Int. J. Heat Technol. 2017, 35, 19–24. [Google Scholar] [CrossRef]
- Stephenson, R.L.; Seaton, S.; McCharen, R.; Hernandez, E.; Pair, R.B. Thermal desorption of oil from oil-based drilling fluids cuttings: Processes and technologies. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Perth, Australia, 14 October 2004. [Google Scholar]
- Muhammed, N.S.; Olayiwola, T.; Elkatatny, S.; Haq, B.; Patil, S. Insights into the application of surfactants and nanomaterials as shale inhibitors for water-based drilling fluid: A review. Nat. Gas Sci. Eng. 2021, 92, 103987. [Google Scholar] [CrossRef]
- Schlemmer, R.; Phoon, G. A new generation associative polymer extends temperature stability of deepwater drilling fluid. In Proceedings of the IPTC 2012: International Petroleum Technology Conference, Bangkok, Thailand, 9 February 2012; p. cp-280-00315. [Google Scholar]
- Edalatfar, M.; Yazdani, F.; Salehi, M.B. Synthesis and identification of ZnTiO3 nanoparticles as a rheology modifier additive in water-based drilling mud. J. Pet. Sci. Eng. 2021, 201, 108415. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Zhang, Y.; Zhong, H.; Huang, W.; Tang, Z. Zwitterionic polymer P (AM-DMC-AMPS) as a low-molecular-weight encapsulator in deepwater drilling fluid. Appl. Sci. 2017, 7, 594. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Sun, J.; Li, Q.; Lv, K.; Wang, J.; Wang, Z. Mechanism of organosilicate polymer as high-temperature resistant inhibitor in water-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128489. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; Chang, X.; Xu, Z.; Zhang, X.; Huang, X.; Liu, J.; Lv, K. Fuels. A novel environment-friendly natural extract for inhibiting shale hydration. Energy Fuels 2019, 33, 7118–7126. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Li, H.; Wang, R.; Lv, K.; Li, H. Fabrication of a Hydrophobic Hierarchical Surface on Shale Using Modified Nano-SiO2 for Strengthening the Wellbore Wall in Drilling Engineering. Engineering 2022, 11, 101–110. [Google Scholar] [CrossRef]
- Sun, J.; Chang, X.; Zhang, F.; Bai, Y.; Lv, K.; Wang, J.; Zhou, X.; Wang, B. Salt-responsive zwitterionic polymer brush based on modified silica nanoparticles as a fluid-loss additive in water-based drilling fluids. Energy Fuels 2020, 34, 1669–1679. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Jin, Z.; Huang, X. The Utilization of Self-Crosslinkable Nanoparticles as High-Temperature Plugging Agent in Water-Based Drilling Fluid. SPE J. 2022, 1–14. [Google Scholar] [CrossRef]
- Sun, J.; Chang, X.; Lv, K.; Wang, J.; Zhang, F.; Zhou, X.; Zhao, J. Salt-responsive zwitterionic copolymer as tackifier in brine drilling fluids. J. Mol. Liq. 2020, 319, 114345. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, G.; Xuan, Y.; Wang, L.; Huang, X. The Development of a Viscosifier for Clay Free and Water Based Drilling Fluid With High Density and High Temperature Resistant. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference, Singapore, 22 August 2016. [Google Scholar]
- Xu, J.-g.; Qiu, Z.; Zhao, X.; Mou, T.; Zhong, H.; Huang, W. A polymer microsphere emulsion as a high-performance shale stabilizer for water-based drilling fluids. RSC Adv. 2018, 8, 20852–20861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, B.; Tchameni, A.P.; Luo, M.; Wen, J. A novel thermo-associating polymer as rheological control additive for bentonite drilling fluid in deep offshore drilling. Mater. Lett. 2021, 284, 128914. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Lv, K.; Liu, J.; Xiu, Z.; Wang, Z.; Huang, X.; Bai, Y.; Wang, J.; Jin, J. Synthesis of hydrophobic associative polymers to improve the rheological and filtration performance of drilling fluids under high temperature and high salinity conditions. J. Pet. Sci. Eng. 2022, 209, 109808. [Google Scholar] [CrossRef]
- Maeda, Y.; Nakamura, T.; Ikeda, I. Changes in the hydration states of poly (N-alkylacrylamide) s during their phase transitions in water observed by FTIR spectroscopy. Macromolecules 2001, 34, 1391–1399. [Google Scholar] [CrossRef]
- Maeda, Y.; Higuchi, T.; Ikeda, I. Change in hydration state during the coil− globule transition of aqueous solutions of poly (N-isopropylacrylamide) as evidenced by FTIR spectroscopy. Langmuir 2000, 16, 7503–7509. [Google Scholar] [CrossRef]
- Du, H.; Qian, X. Molecular dynamics simulations of PNIPAM-co-PEGMA copolymer hydrophilic to hydrophobic transition in NaCl solution. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1112–1122. [Google Scholar] [CrossRef]
- Xie, B.; Ting, L.; Zhang, Y.; Liu, C. Rheological properties of bentonite-free water-based drilling fluids with novel polymer viscosifier. J. Pet. Sci. Eng. 2018, 164, 302–310. [Google Scholar] [CrossRef]
- Jiang, G.; Sun, J.; He, Y.; Cui, K.; Dong, T.; Yang, L.; Yang, X.; Wang, X. Novel water-based drilling and completion fluid technology to improve wellbore quality during drilling and protect unconventional reservoirs. Engineering 2021, in press. [Google Scholar] [CrossRef]
















| Parameter Ratios | BF | BF + 0.2 wt% XC | BF + 0.2 wt% PNABAM |
|---|---|---|---|
| AV4°C:AV25°C | 2.23 | 1.72 | 1.35 |
| AV4°C:AV65°C | 4.75 | 3.31 | 2.25 |
| PV4°C:PV25°C | 2.14 | 1.77 | 1.22 |
| PV4°C:PV65°C | 5.00 | 3.20 | 2.44 |
| YP4°C:YP25°C | 2.67 | 1.57 | 2.5 |
| YP4°C:YP65°C | 4.00 | 3.67 | 1.67 |
| Conditions | Density/g/cm3 | AV/mPa·s | PV/mPa·s | YP/Pa | G′/G″Pa | FLAPI/mL | FLHTHP/mL |
|---|---|---|---|---|---|---|---|
| Before ageing | 1.5 | 45 | 36 | 9 | 8/11 | 4 | - |
| After 120 °C/16 h ageing | 1.5 | 62 | 44 | 18 | 3/8 | 4.4 | 10.4 |
| Parameter Ratios | WBDF-1 | WBDF-2 |
|---|---|---|
| AV4°C:AV25°C | 1.36 | 1.27 |
| AV4°C:AV65°C | 1.93 | 1.81 |
| PV4°C:PV25°C | 1.40 | 1.19 |
| PV4°C:PV65°C | 1.92 | 1.68 |
| YP4°C:YP25°C | 1.14 | 1.42 |
| YP4°C:YP65°C | 2.00 | 2.06 |
| Temperature/°C | Particle Size | Particle Size Distribution Graph |
|---|---|---|
| 25 | D10 = 177 nm D50 = 255 nm Dave = 337.4 nm |  |
| 30 | D10 = 205 nm D50 = 648 nm Dave = 593.8 nm |  |
| 35 | D10 = 1050 nm D50 = 1330 nm Dave = 1241 nm |  |
| Component | Additions |
|---|---|
| water | 400 mL |
| bentonite | 16 g |
| Na2CO3 | 1.2 g |
| Component | Function | Mass Fraction/wt% | |
|---|---|---|---|
| WBDF-1 | WBDF-2 | ||
| bentonite | filtrate reducer and viscosifier | 4 | 4 |
| PNBAM | rheology modifier | 0 | 0.2 |
| XC | viscosifier | 0.2 | 0 |
| PAC-LV | filtrate reducer | 1.5 | 1.5 |
| AP-1 | shale inhibitor | 1 | 1 |
| Methyl oleate | lubricants | 2 | 2 |
| NaCl | hydrate inhibitor | 20 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Sun, J.; Zhang, K.; Lv, K.; Huang, X.; Wang, J.; Wang, R.; Meng, X. A Temperature-Sensitive Polymeric Rheology Modifier Used in Water-Based Drilling Fluid for Deepwater Drilling. Gels 2022, 8, 338. https://doi.org/10.3390/gels8060338
Wang Z, Sun J, Zhang K, Lv K, Huang X, Wang J, Wang R, Meng X. A Temperature-Sensitive Polymeric Rheology Modifier Used in Water-Based Drilling Fluid for Deepwater Drilling. Gels. 2022; 8(6):338. https://doi.org/10.3390/gels8060338
Chicago/Turabian StyleWang, Zhongyi, Jinsheng Sun, Kun Zhang, Kaihe Lv, Xianbin Huang, Jintang Wang, Ren Wang, and Xu Meng. 2022. "A Temperature-Sensitive Polymeric Rheology Modifier Used in Water-Based Drilling Fluid for Deepwater Drilling" Gels 8, no. 6: 338. https://doi.org/10.3390/gels8060338